Re-Evaluation of Forest Biomass Carbon Stocks and Lessons from the World’S Most Carbon-Dense Forests
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
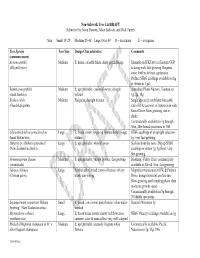
Recommended Non-Sidewalk Tree List DRAFT
Non-Sidewalk Tree List DRAFT Submitted by Jason Dewees, Mike Sullivan, and Dick Turner Size: Small 15-25’ Medium 25-40’ Large Over 40’ D = deciduous E = evergreen Tree Species Tree Size Design Characteristics Comments (common name) Acmena smithii Medium E; dense, colorful fruits; shiny green foliage Example on JFK Drive in Eastern GGP (lilly-pilly tree) is doing well; fast-growing. Requires some fertility for best appearance. Profuse SFBG seedlings available to dig or obtain in 1 gal. Banksia integrifolia Medium E; upright habit; creamy flowers; drought Australian Plants Nursery, Ventura, in (coast banksia) tolerant 1g, 5g, 15g Brahea edulis Medium Fan palm; drought tolerant Single specimen established on north (Guadalupe palm) side of JFK just west of intersection with Kezar Drive. Slow growing, sun or shade. Commercially available in 1g through 36in, 48in boxed specimens to 15ft Chiranthodendron pentadactylon Large E; broad crown; large red flowers, bold foliage SFBG seedlings of an upright selection: (hand flower tree) texture 1g; very fast-growing Hoheria sp. (Hoheria populnea? Large E; upright habit; white flowers Suckers from the roots. Dig up SFBG (New Zealand lacebark)) seedlings or obtain 1g, 5g there; very fast-growing Hymenosporum flavum Medium E; upright habit; yellow flowers; fast growing Boething, Valley Crest; commercially (sweetshade) available in 24in & 36in; fast-growing. Jubaea chilensis Large Feather palm; broad crown of leaves; silvery Magnificent specimen at JFK & Fuchsia (Chilean palm) trunk; sun-loving Drive; drought-tolerant, prefers sun. Slow-growing until trunking phase, then moderate growth speed. Commercially available in 5g through 20ft B&B specimens Leptospermum scoparium ‘Helene Small E; broad, low crown; pink flowers; often multi- Suncrest Nurseries 5g Strybing’ (New Zealand tea tree) trunked Metrosideros robusta Large E; broad dense crown; showy red flowers in SFBG Nursery seedlings available in 1g (northern rata) summer; slow & unusual but very well-adapted Michelia/Magnolia champaca or M. -

Failing to Conserve Leadbeater's Possum and Its Mountain Ash Forest
CORE Metadata, citation and similar papers at core.ac.uk Provided by The Australian National University Failing to conserve Leadbeater’s Possum and its Mountain Ash forest habitat David Blair1, David Lindenmayer1 &Lachlan McBurney1 1Fenner School of Environment and Society, The Australian National University, Canberra, ACT 2601 Corresponding author: [email protected] The conservation of the Critically Endangered Leadbeater’s Possum Gymnobelideus leadbeateri in Victoria’s Mountain Ash Eucalyptus regnans forests is one of the most controversial native mammal conservation issues in Australia. Much of the controversy results from long-running conflicts between the demands of the native forest logging industry and associated impacts on Leadbeater’s Possum and its Mountain Ash forest habitat. Here we argue that despite a legislative obligation to protect Leadbeater’s Possum and some limited recent improvements in management, conservation efforts for the species have gone backwards over the past decade. The key problems we identify include that the Victorian Government has: (1) maintained levels of wood production that are too high given the amount of the forest estate that was burned in 2009, (2) failed to substitute clearfell logging practices with more ecologically-sensitive Variable Retention Harvesting Systems, (3) ignored the science (including by its own researchers) on the need for a large protected area for Leadbeater’s Possum, (4) altered key definitions such as those for mature trees and old growth that have substantially weakened the ability to protect Leadbeater’s Possum, and (5) overlooked the array of forest values beyond timber production (such as water and tourism) and which make a greater contribution to the economy. -

Vicforests' Koala Management
VicForests Instruction Koala Management August 2015 1.0 Copyright © VicForests All rights reserved. No part of this document may be reproduced, stored in a retrieval system, or transmitted in any form or by any means electronic, mechanical, photocopying, recording or otherwise, without prior written permission of VicForests. Document Information General Information File Path : J:\VICFORESTS\PUBLIC\SUST. FOREST. MAN. SYSTEM\ FOREST OPERATIONS MANUAL\WRITTEN INSTRUCTIONS\APPROVED\VICFORESTS INSTRUCTION – KOALA MANAGEMENT RecFind Ref: Description: This document outlines VicForests process for managing Koalas Author: C. Powell Creation Date: July 2015 Procedure Owner(s): Conservation Biologist Current Version: 1.0 Copy Number: 1 Review Period: Last review date: 5/08/2015 Next review date: 5/08/2018 Revision History New Version Revision Date Author(s) Old Version Revision Notes Reviewers The following positions should review the instruction prior to any significant amendment being approved General Manager Planning Manager Forest Performance Approval Approver Position / Resolution Date Nathan Trushell General Manager, Planning Signature: Unless stamped ‘CONTROLLED COPY’ in red, all printed copies of this procedure are uncontrolled. The latest version can be found in VicForests SharePoint– Sustainable Forest Management System Version: 1.0 Last Updated: 5/08/15 Procedure Owner: Conservation Biologist Page 2 of 9 TABLE OF CONTENTS 1. Purpose .................................................................................................................................... -

Observations on a Nesting Hollow of Yellow- Tailed Black Cockatoo, and the Felled Tree That Hosted It, in North-Eastern Tasmania
59 The Tasmanian Naturalist (2004) 126: 59-63 OBSERVATIONS ON A NESTING HOLLOW OF YELLOW- TAILED BLACK COCKATOO, AND THE FELLED TREE THAT HOSTED IT, IN NORTH-EASTERN TASMANIA Mark Wapstra1 and Niall Doran2 1Forest Practices Board, 30 Patrick Street, Hobart, Tasmania 7000 Email: [email protected] 2Threatened Species Unit, Department of Primary Industries, Water & Environment, GPO Box 44, Hobart, Tasmania 7001 INTRODUCTION The yellow-tailed black cockatoo (Calyptorhynchus funereus Shaw, 1794) is one of Tasmania’s most familiar birds, its common name aptly describing its distinctive black plumage with yellow undertail. The species is gregarious and is usually seen in family groups or small parties and occasionally congregates in large flocks (Forshaw and Cooper, 1981). In north-eastern Tasmania, large flocks are a familiar sight and sound in areas of extensive softwood plantation. The yellow-tailed black cockatoo is native to Tasmania and is widely distrib- uted throughout the State (Brown and Holdsworth, 1992). It is nomadic and cov- ers large distances in search for food, which comprises seeds, nuts, fruit or ber- ries from a wide range of native trees and shrubs such as eucalypts, banksias, acacias and hakea but also a large range of insects and larvae, and seeds and nuts of introduced flora such as pines. There are few reported observations of breeding behaviour of the yellow- tailed black cockatoo in Tasmania (Brown and Holdsworth, 1992) but the spe- cies is known to use large hollows in over-mature (often dead) eucalypts, in (primarily wet) sclerophyll forests. Haseler and Taylor (1993) provide informa- tion on a nest tree from dry sclerophyll forest in north-eastern Tasmania. -

Eucalyptus Species for Taranaki
Eucalyptus Species for Taranaki 14 Introduction conditions. Especially suited to saline winds. This This information sheet follows on from the information species holds its form, mills extremely well at a young sheet, ‘Eucalyptus’ (No.13), which discusses general age, and is largely unaffected by pests and diseases. management issues such as siting, selecting tree stocks, Eucalyptus nitens shining gum E. nitens is more tolerant planting regimes, silviculture, establishment, weed to wet sites and is suited to planting in all damper sites control, planting technique, fertiliser requirements, and that E. fraxinoides won't tolerate, for example, low lying pest and disease control. damper areas along streambanks and on hillsites affected by springs. It is also equally suited to drier As no one species of eucalypt will thrive over the range 'fraxinoides' sites. Generally, E. nitens is suited to of sites in a similar manner to Pinus radiata, selecting the planting in soils that are a bit damper than pine will most suitable species for a particular site is of critical tolerate. Furthermore, the tree has good form, a fast importance. Species selection is just as important, if not growth rate, and is resistant to cold. It has a good more, than issues associated with their subsequent reputation for milling and exceptional peeling management. properties (better than radiata pine), although more trial work on drying properties is required. E. nitens A lack of objective, accessible, practical local knowledge used to be affected by the paropsis tortoise beetle and experience of eucalypt growing in Taranaki makes (Paropsis charybdis), but since that beetle has been it difficult for people seeking advice on correct species controlled, the species is largely free of pest and disease to plant. -

Agathis Robusta and Agathis Australis Friends Friends
Plants in Focus, December 2016 Agathis robusta and Agathis australis Friends of GeelongBotanic Left: The Qld Kauri Agathis robusta, planted in the Albury BG in 1910, is the largest recorded in the Big Tree Register. Note gardener. [1] Right: The NZ Kauri Agathis australis, named Tane Mahuta (Lord of the Forest), in the Waipoua Forest is the largest known in NZ. Photo: Prof. Chen Hualin, CC BY-SA 4.0, zh.wikipedia.org Kauris (Agathis sp.) are conifers Conifers, along with the other Gymnosperms (Cycads and Ginkgoes), first appeared about 300 Ma (Million years ago) at the end of the Carboniferous when the world’s coal deposits were being laid down with the remains of the spore-producing trees of that period. The early conifers looked like modern Araucaria. These trees spread throughout the world and displaced their predecessors. The age of the seed plants had arrived. The conifers are a hardy lot. They survived the largest mass extinction the earth has known, 252 Ma, at the end of the Permian Period. But more challenges lay ahead. Sometime in the next 50 Myr (Million years) one of Gymnosperms gave rise to the flowering plants, the Angiosperms. By 100 Ma, in the Cretaceous period, Angiosperms were widespread. And so the battle began - and still continues to this day. The flowering plants have many features that make them more successful in many environments, so their take-over of many habitats was complete by about 65 Ma at the end of the age of the dinosaurs. But in the world’s harsh environments the conifers continue to not just survive, but flourish. -

Eucalyptus Species Trials on Pumiceland
Eucalyptus species trials on pumiceland G.R. Johnson and M.D. Wilcox Trial Sites All three trial sites were flat but otherwise varied as follows: ABSTRACT Rotoehu: Warm site, altitude 70 m. Former pasture on a sandy pumice soil. The site was rotary hoed before Twenty species and two hybrids of Eucalyptus were tested being planted in November 1977. on three central North Island pumice/and sites at altitudes of ?Om, 380 m, and 920 m. At age nine years Eucalyptus Waiotapu: Intermediate in temperature, altitude 380 m. A sallgna had performed the best on the warmer low altitude former firebreak on a hydrothermal mud soil site, E. delegatensis and E. dendromorpha had per depleted of topsoil. Planting lines were ripped as a formed well on the high altitude site, and E. regnans was form of soil cultivation before planting in the st on the i'1:termdiate altitude site. For overall adap November 1977. tability on pumice soils E. regnans and E. fastigata were Matea: Cold site, altitude 920 m. Formerly in scrub of the best, but E. delegatensis and E. fraxinoides also did Leptospermum, Dracophyllum, Phyllocladus, and well on more than one site. Eucalyptus nitens showed Hebe. Humic topsoil overlies a yellow pumice at excellent potential on all three sites, notwithstanding its this site. Before planting in December of 1977 the susceptibility to Paropsis attack. area was: crushed (July, 1976), burnt (December, 1976) disced and ripped (May, 1977), and sprayed 'Introduction with atrazine/amitrole and simazine (August, Eucalyptus have been planted in New Zealand for over 100 1977). -

Myrtle Rust Reviewed the Impacts of the Invasive Plant Pathogen Austropuccinia Psidii on the Australian Environment R
Myrtle Rust reviewed The impacts of the invasive plant pathogen Austropuccinia psidii on the Australian environment R. O. Makinson 2018 DRAFT CRCPLANTbiosecurity CRCPLANTbiosecurity © Plant Biosecurity Cooperative Research Centre, 2018 ‘Myrtle Rust reviewed: the impacts of the invasive pathogen Austropuccinia psidii on the Australian environment’ is licenced by the Plant Biosecurity Cooperative Research Centre for use under a Creative Commons Attribution 4.0 Australia licence. For licence conditions see: https://creativecommons.org/licenses/by/4.0/ This Review provides background for the public consultation document ‘Myrtle Rust in Australia – a draft Action Plan’ available at www.apbsf.org.au Author contact details R.O. Makinson1,2 [email protected] 1Bob Makinson Consulting ABN 67 656 298 911 2The Australian Network for Plant Conservation Inc. Cite this publication as: Makinson RO (2018) Myrtle Rust reviewed: the impacts of the invasive pathogen Austropuccinia psidii on the Australian environment. Plant Biosecurity Cooperative Research Centre, Canberra. Front cover: Top: Spotted Gum (Corymbia maculata) infected with Myrtle Rust in glasshouse screening program, Geoff Pegg. Bottom: Melaleuca quinquenervia infected with Myrtle Rust, north-east NSW, Peter Entwistle This project was jointly funded through the Plant Biosecurity Cooperative Research Centre and the Australian Government’s National Environmental Science Program. The Plant Biosecurity CRC is established and supported under the Australian Government Cooperative Research Centres Program. EXECUTIVE SUMMARY This review of the environmental impacts of Myrtle Rust in Australia is accompanied by an adjunct document, Myrtle Rust in Australia – a draft Action Plan. The Action Plan was developed in 2018 in consultation with experts, stakeholders and the public. The intent of the draft Action Plan is to provide a guiding framework for a specifically environmental dimension to Australia’s response to Myrtle Rust – that is, the conservation of native biodiversity at risk. -

26 Extreme Trees Pub 2020
Publication WSFNR-20-22C April 2020 Extreme Trees: Tallest, Biggest, Oldest Dr. Kim D. Coder, Professor of Tree Biology & Health Care / University Hill Fellow University of Georgia Warnell School of Forestry & Natural Resources Trees have a long relationship with people. They are both utility and amenity. Trees can evoke awe, mysticism, and reverence. Trees represent great public and private values. Trees most noticed and celebrated by people and communities are the one-tenth of one-percent of trees which approach the limits of their maximum size, reach, extent, and age. These singular, historic, culturally significant, and massive extreme trees become symbols and icons of life on Earth, and our role model in environmental stewardship and sustainability. What Is A Tree? Figure 1 is a conglomeration of definitions and concepts about trees from legal and word definitions in North America. For example, 20 percent of all definitions specifically state a tree is a plant. Concentrated in 63% of all descriptors for trees are four terms: plant, woody, single stem, and tall. If broad stem diameter, branching, and perennial growth habit concepts are added, 87% of all the descriptors are represented. At its most basic level, defining a tree is not species based, but is a structural definition. A tree is represented by a type of plant architecture recognizable by non-technical people. The most basic concepts for defining a tree are — a large, tall, woody, perennial plant with a single, unbranched, erect, self-supporting stem holding an elevated and distinct crown of branches, and which is greater than 10 feet in height and greater than 3 inches in diameter. -

NOTES on AGATHIS AUSTRALIS. by C
NOTES ON AGATHIS AUSTRALIS. By C. T. SANDO. Introduction.—Much has been published about our kauri (Agathis australis) and it is now generally recognised as one of the finest coniferous trees of the world. The New Zealand species is one of a genus of ten—the most important being— A. Palmerstoni ... (Queensland) A. microstachya ... (Queensland) A. robusta ... (Queensland and Philippine Islands) A. vitiensis * ... (Fiji) A. alba ... (Malaya, Sumatra, Java, Celebes, Borneo, and Philippine Islands) A. lanceolata ... (New Caledonia) A. macrophylla ... (Solomon Islands) The value of Agathis australis was first recognised before 1800 by which time a considerable trade in kauri spars had sprung up between Australia and New Zealand. Later, naval-store ships were sent out from England for cargoes and from that time on kauri has increased in value. Up till a short time ago it was used for building and interior furnishing, but now the limited supplies demand a price far too high for these purposes so that its present uses have been confined to carriage building, cabinet-making, and general joinery, vats, tubs, and other special purposes. Wanton waste has so depleted out kauri areas that now, more than ever it is forced upon us, the necessity of making very serious attempts at conservation of the remaining supplies and propagation of this tree for scenic purposes, and, if economic conditions warrant it, for timber production. Geographical, Climatic, and Edaphic Range.—Kauri occurs in the northern parts of the North Island except in the extreme northern peninsula. It is found from Ahipara and Mangonui in the north as far south as the Bay of Plenty on the East Coast and Kawhia Harbour on the west, i.e. -

The Natural Distribution of Eucalyptus Species in Tasmania
The natural distribution of Eucalyptus species in Tasmania K.J. Williams and B.M. Potts Cooperative Research Centre for Temperate Hardwood Forestry, Department of Plant Science, University of Tasmania, GPO Box 252–55, Hobart 7001 email: [email protected]./[email protected] Abstract dispersed (E. cordata) or disjunct (E. archeri) occurrences. Most species that are rare in A summary is provided of the natural geographic Tasmania are endemics, with the exception of distributions of the 29 Tasmanian Eucalyptus E. perriniana and E. aff. radiata, although species. The work is based on over 60 000 the taxonomic status of the latter requires observations from numerous data sources. A map investigation. Unresolved issues relating to the on a 10 km x 10 km grid-cell scale is presented for natural distribution and taxonomic affinities of each species and is accompanied by graphs of the the Tasmanian eucalypt species are summarised. altitudinal range and flowering times, as well as descriptive notes on distribution and ecology, supplemented with a list of key references. The Introduction geographic pattern of species richness is examined at generic, subgeneric and series levels. Total In Tasmania and the Bass Strait islands, species richness is greater in the drier, eastern 29 native eucalypt species (one of which has regions compared to the wet, western regions of two subspecies) are recognised by Buchanan Tasmania, with highest concentrations of species (1995), from two informal subgenera, occurring mainly in the central east coast and Monocalyptus and Symphyomyrtus (Pryor and south-eastern regions. Monocalyptus species Johnson 1971). -

The Scientific Reserves of Auckland University. Ii. Quantitative Vegetation Studies
TANE 29, 1983 THE SCIENTIFIC RESERVES OF AUCKLAND UNIVERSITY. II. QUANTITATIVE VEGETATION STUDIES by John Ogden Department of Botany, University of Auckland, Private Bag, Auckland SUMMARY Forest vegetation surveys, using both plots and point-centred-quarter (plotless) methods, were undertaken by students in the University reserves at Swanson, Huapai, Oratia and Leigh over the period 1979 to 1982. The two methods gave similar estimates of species composition, total basal area and total density, but both gave wide confidence intervals on the parameters being estimated. The "leading species", in terms of both density and basal area, are defined for each area. The differences between the rankings for these different vegetation parameters are commented upon A regression of age on height of kauri (Agathis australis) seedlings is presented. The relationship between age and trunk diameter is discussed. It is shown that in cores taken from kauri poles in ricker stands over the size range 10 to 40 cm DBH the relationship between age and diameter is very weak. When a wider size range is employed a highly statistically significant relationship is obtained. It is concluded that the highly skewed size class frequency distribution of kauri, characteristic of such stands, reflects a competitive hierarchy. The senile population structure of kanuka (Leptospermum ericoides) in ricker stands confirms their serai role in forest development. The basal area estimates are discussed in the context of other figures for New Zealand forests. It is concluded that although the values are high (average 70 m2 ha 1) they are nevertheless below those commonly found in mature kauri forest.