Phylogenetic Relationships and Time-Calibration of the South American Fossil and Extant Species of Southern Beeches (Nothofagus)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
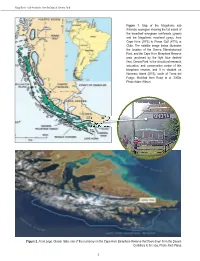
Chapter14.Pdf
PART I • Omora Park Long-Term Ornithological Research Program THE OMORA PARK LONG-TERM ORNITHOLOGICAL RESEARCH PROGRAM: 1 STUDY SITES AND METHODS RICARDO ROZZI, JAIME E. JIMÉNEZ, FRANCISCA MASSARDO, JUAN CARLOS TORRES-MURA, AND RAJAN RIJAL In January 2000, we initiated a Long-term Ornithological Research Program at Omora Ethnobotanical Park in the world's southernmost forests: the sub-Antarctic forests of the Cape Horn Biosphere Reserve. In this chapter, we first present some key climatic, geographical, and ecological attributes of the Magellanic sub-Antarctic ecoregion compared to subpolar regions of the Northern Hemisphere. We then describe the study sites at Omora Park and other locations on Navarino Island and in the Cape Horn Biosphere Reserve. Finally, we describe the methods, including censuses, and present data for each of the bird species caught in mist nets during the first eleven years (January 2000 to December 2010) of the Omora Park Long-Term Ornithological Research Program. THE MAGELLANIC SUB-ANTARCTIC ECOREGION The contrast between the southwestern end of South America and the subpolar zone of the Northern Hemisphere allows us to more clearly distinguish and appreciate the peculiarities of an ecoregion that until recently remained invisible to the world of science and also for the political administration of Chile. So much so, that this austral region lacked a proper name, and it was generally subsumed under the generic name of Patagonia. For this reason, to distinguish it from Patagonia and from sub-Arctic regions, in the early 2000s we coined the name “Magellanic sub-Antarctic ecoregion” (Rozzi 2002). The Magellanic sub-Antarctic ecoregion extends along the southwestern margin of South America between the Gulf of Penas (47ºS) and Horn Island (56ºS) (Figure 1). -

Bioclimatic and Phytosociological Diagnosis of the Species of the Nothofagus Genus (Nothofagaceae) in South America
International Journal of Geobotanical Research, Vol. nº 1, December 2011, pp. 1-20 Bioclimatic and phytosociological diagnosis of the species of the Nothofagus genus (Nothofagaceae) in South America Javier AMIGO(1) & Manuel A. RODRÍGUEZ-GUITIÁN(2) (1) Laboratorio de Botánica, Facultad de Farmacia, Universidad de Santiago de Compostela (USC). E-15782 Santiago de Com- postela (Galicia, España). Phone: 34-881 814977. E-mail: [email protected] (2) Departamento de Producción Vexetal. Escola Politécnica Superior de Lugo-USC. 27002-Lugo (Galicia, España). E-mail: [email protected] Abstract The Nothofagus genus comprises 10 species recorded in the South American subcontinent. All are important tree species in the ex- tratropical, Mediterranean, temperate and boreal forests of Chile and Argentina. This paper presents a summary of data on the phyto- coenotical behaviour of these species and relates the plant communities to the measurable or inferable thermoclimatic and ombrocli- matic conditions which affect them. Our aim is to update the phytosociological knowledge of the South American temperate forests and to assess their suitability as climatic bioindicators by analysing the behaviour of those species belonging to their most represen- tative genus. Keywords: Argentina, boreal forests, Chile, mediterranean forests, temperate forests. Introduction tually give rise to a temperate territory with rainfall rates as high as those of regions with a Tropical pluvial bio- The South American subcontinent is usually associa- climate; iii. finally, towards the apex of the American ted with a tropical environment because this is in fact the Southern Cone, this temperate territory progressively dominant bioclimatic profile from Panamá to the north of gives way to a strip of land with a Boreal bioclimate. -

This Article Appeared in a Journal Published by Elsevier. the Attached
This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues. Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited. In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier’s archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/copyright Author's personal copy Palaeogeography, Palaeoclimatology, Palaeoecology 280 (2009) 480–488 Contents lists available at ScienceDirect Palaeogeography, Palaeoclimatology, Palaeoecology journal homepage: www.elsevier.com/locate/palaeo Early Miocene subtropical water temperatures in the southeast Pacific Sven N. Nielsen ⁎,1, Johannes Glodny Deutsches GeoForschungsZentrum (GFZ), Telegrafenberg, 14473 Potsdam, Germany article info abstract Article history: Cenozoic climate of western South America is strongly controlled by features like Andean uplift and the Received 14 May 2009 Humboldt Current. The first strontium isotope age data from central and southern Chile provide a latest Accepted 24 June 2009 Oligocene to late early Miocene age for classic warm-water mollusk faunas reaching as far south as 45°S. Available online 3 July 2009 Comparison with the biogeography of congeneric living species indicates that sea surface temperatures off central and southern Chile during that time were at least 5 °C higher than today; i.e., minimum annual mean Keywords: sea surface temperatures for Darwin's Navidad fauna at 34°S are estimated as 20 °C. -

Development and Evaluation of Rrna Targeted in Situ Probes and Phylogenetic Relationships of Freshwater Fungi
Development and evaluation of rRNA targeted in situ probes and phylogenetic relationships of freshwater fungi vorgelegt von Diplom-Biologin Christiane Baschien aus Berlin Von der Fakultät III - Prozesswissenschaften der Technischen Universität Berlin zur Erlangung des akademischen Grades Doktorin der Naturwissenschaften - Dr. rer. nat. - genehmigte Dissertation Promotionsausschuss: Vorsitzender: Prof. Dr. sc. techn. Lutz-Günter Fleischer Berichter: Prof. Dr. rer. nat. Ulrich Szewzyk Berichter: Prof. Dr. rer. nat. Felix Bärlocher Berichter: Dr. habil. Werner Manz Tag der wissenschaftlichen Aussprache: 19.05.2003 Berlin 2003 D83 Table of contents INTRODUCTION ..................................................................................................................................... 1 MATERIAL AND METHODS .................................................................................................................. 8 1. Used organisms ............................................................................................................................. 8 2. Media, culture conditions, maintenance of cultures and harvest procedure.................................. 9 2.1. Culture media........................................................................................................................... 9 2.2. Culture conditions .................................................................................................................. 10 2.3. Maintenance of cultures.........................................................................................................10 -

Precise Age and Biostratigraphic Significance of the Kinney Brick Quarry Lagerstätte, Pennsylvanian of New Mexico, USA
Precise age and biostratigraphic significance of the Kinney Brick Quarry Lagerstätte, Pennsylvanian of New Mexico, USA Spencer G. Lucas1, Bruce D. Allen2, Karl Krainer3, James Barrick4, Daniel Vachard5, Joerg W. Schneider6, William A. DiMichele7 and Arden R. Bashforth8 1New Mexico Museum of Natural History, 1801 Mountain Road N.W., Albuquerque, New Mexico, 87104, USA email: [email protected] 2New Mexico Bureau of Geology and Mineral Resources, 801 Leroy Place, Socorro, New Mexico, 87801, USA email: [email protected] 3Institute of Geology and Paleontology, University of Innsbruck, Innsbruck, A-6020, Austria email: [email protected] 4Department of Geosciences, Texas Tech University, Box 41053, Lubbock, Texas, 79409, USA email: [email protected] 5Université des Sciences et Technologies de Lille, UFR des Sciences de la Terre, UPRESA 8014 du CNRS, Laboratoire LP3, Bâtiment SN 5, F-59655 Villeneuve d’Ascq, Cédex, France email: [email protected] 6TU Bergakademie Freiberg, Cottastasse 2, D-09596 Freiberg, Germany email:[email protected] 7Department of Paleobiology, NMNH Smithsonian Institution, Washington, DC 20560 email: [email protected] 8Geological Museum, Natural History Museum of Denmark, University of Copenhagen, Øster Voldgade 5-7, 1350 Copenhagen K, Denmark email: [email protected] ABSTRACT: The Kinney Brick Quarry is a world famous Late Pennsylvanian fossil Lagerstätte in central New Mexico, USA. The age assigned to the Kinney Brick Quarry (early-middle Virgilian) has long been based more on its inferred lithostratigraphic position than on biostratigraphic indicators at the quarry. We have developed three datasets —-stratigraphic position, fusulinids and conodonts— that in- dicate the Kinney Brick Quarry is older, of middle Missourian (Kasimovian) age. -

A Forgotten Kingdom Ecologically Industrious and Alluringly Diverse, Australia’S Puffballs, Earthstars, Jellies, Agarics and Their Mycelial Kin Merit Your Attention
THE OTHER 99% – NEGLECTED NATURE The delicate umbrellas of this Mycena species last only fleetingly, while its fungal mycelium persists, mostly obscured within the log it is rotting. Photo: Alison Pouliot A Forgotten Kingdom Ecologically industrious and alluringly diverse, Australia’s puffballs, earthstars, jellies, agarics and their mycelial kin merit your attention. Ecologist Alison Pouliot ponders our bonds with the mighty fungus kingdom. s the sun rises, I venture off-track Fungi have been dubbed the ‘forgotten into a dripping forest in the Otway kingdom’ – their ubiquity and diversity ARanges. Mountain ash tower contrast with the sparseness of knowledge overhead, their lower trunks carpeted about them, they are neglected in in mosses, lichens and liverworts. The conservation despite their ecological leeches are also up early and greet me significance, and their aesthetic and with enthusiasm. natural history fascination are largely A white scallop-shaped form at the unsung in popular culture. The term base of a manna gum catches my eye. ‘flora and fauna’ is usually unthinkingly Omphalotus nidiformis, the ghost fungus. A assumed to cover the spectrum of visible valuable marker. If it’s dark when I return, life. I am part of a growing movement of the eerie pale green glow of this luminous fungal enthusiasts dedicated to lifting fungal cairn will be a welcome beacon. the profile of the ‘third f’ in science, Descending deeper into the forest, a conservation and society. It is an damp funk hits my nostrils, signalling engrossing quest, not only because of the fungi. As my eyes adjust and the morning alluring organisms themselves but also for lightens, I make out diverse fungal forms the curiosities of their social and cultural in cryptic microcosms. -

Dynamics of Even-Aged Nothofagus Truncata and N. Fusca Stands in North Westland, New Zealand
12 DYNAMICS OF EVEN-AGED NOTHOFAGUS TRUNCATA AND N. FUSCA STANDS IN NORTH WESTLAND, NEW ZEALAND M. C SMALE Forest Research Institute, Private Bag, Rotorua, New Zealand H. VAN OEVEREN Agricultural University, Salverdaplein 11, P.O. Box 9101, 6700 HB, Wageningen, The Netherlands C D. GLEASON* New Zealand Forest Service, P.O. Box 138, Hokitika, New Zealand and M. O. KIMBERLEY Forest Research Institute, Private Bag, Rotorua, New Zealand (Received for publication 30 August, 1985; revision 12 January 1987) ABSTRACT Untended, fully stocked, even-aged stands of Nothofagus truncata (Col.) Ckn. (hard beech) or N. fusca (Hook, f.) Oerst. (red beech) of natural and cultural origin and ranging in age from 20 to 100 years, were sampled using temporary and permanent plots on a range of sites in North Westland, South Island, New Zealand. Changes in stand parameters with age were quantified in order to assess growth of these stands, and thus gain some insight into their silvicultural potential. Stands of each species followed a similar pattern of growth, with rapid early height and basal area increment. Mean top height reached a maximum of c. 27 m by age 100 years. Basal area reached an equilibrium of c. 41 m2/ha in N. truncata and 46 m2/ha in N. fusca as early as age 30 years. Nothofagus truncata stands had, on average, a somewhat lower mean diameter at any given age than N. fusca stands, and maintained higher stockings. Both species attained similar maximum volume of c. 460 m3/ha at age 100 years. Keywords: even-aged stands; stand dynamics; growth; Nothofagus truncata; Nothofagus fusca. -

Impact of Extreme Weather Events on Aboveground Net Primary Productivity and Sheep Production in the Magellan Region, Southernmost Chilean Patagonia
geosciences Article Impact of Extreme Weather Events on Aboveground Net Primary Productivity and Sheep Production in the Magellan Region, Southernmost Chilean Patagonia Pamela Soto-Rogel 1,* , Juan-Carlos Aravena 2, Wolfgang Jens-Henrik Meier 1, Pamela Gross 3, Claudio Pérez 4, Álvaro González-Reyes 5 and Jussi Griessinger 1 1 Institute of Geography, Friedrich–Alexander-University of Erlangen–Nürnberg, 91054 Erlangen, Germany; [email protected] (W.J.-H.M.); [email protected] (J.G.) 2 Centro de Investigación Gaia Antártica, Universidad de Magallanes, Punta Arenas 6200000, Chile; [email protected] 3 Servicio Agrícola y Ganadero (SAG), Punta Arenas 6200000, Chile; [email protected] 4 Private Consultant, Punta Arenas 6200000, Chile; [email protected] 5 Hémera Centro de Observación de la Tierra, Escuela de Ingeniería Forestal, Facultad de Ciencias, Universidad Mayor, Camino La Pirámide 5750, Huechuraba, Santiago 8580745, Chile; [email protected] * Correspondence: [email protected] Received: 28 June 2020; Accepted: 13 August 2020; Published: 16 August 2020 Abstract: Spatio-temporal patterns of climatic variability have effects on the environmental conditions of a given land territory and consequently determine the evolution of its productive activities. One of the most direct ways to evaluate this relationship is to measure the condition of the vegetation cover and land-use information. In southernmost South America there is a limited number of long-term studies on these matters, an incomplete network of weather stations and almost no database on ecosystems productivity. In the present work, we characterized the climate variability of the Magellan Region, southernmost Chilean Patagonia, for the last 34 years, studying key variables associated with one of its main economic sectors, sheep production, and evaluating the effect of extreme weather events on ecosystem productivity and sheep production. -

Museum of Economic Botany, Kew. Specimens Distributed 1901 - 1990
Museum of Economic Botany, Kew. Specimens distributed 1901 - 1990 Page 1 - https://biodiversitylibrary.org/page/57407494 15 July 1901 Dr T Johnson FLS, Science and Art Museum, Dublin Two cases containing the following:- Ackd 20.7.01 1. Wood of Chloroxylon swietenia, Godaveri (2 pieces) Paris Exibition 1900 2. Wood of Chloroxylon swietenia, Godaveri (2 pieces) Paris Exibition 1900 3. Wood of Melia indica, Anantapur, Paris Exhibition 1900 4. Wood of Anogeissus acuminata, Ganjam, Paris Exhibition 1900 5. Wood of Xylia dolabriformis, Godaveri, Paris Exhibition 1900 6. Wood of Pterocarpus Marsupium, Kistna, Paris Exhibition 1900 7. Wood of Lagerstremia parviflora, Godaveri, Paris Exhibition 1900 8. Wood of Anogeissus latifolia , Godaveri, Paris Exhibition 1900 9. Wood of Gyrocarpus jacquini, Kistna, Paris Exhibition 1900 10. Wood of Acrocarpus fraxinifolium, Nilgiris, Paris Exhibition 1900 11. Wood of Ulmus integrifolia, Nilgiris, Paris Exhibition 1900 12. Wood of Phyllanthus emblica, Assam, Paris Exhibition 1900 13. Wood of Adina cordifolia, Godaveri, Paris Exhibition 1900 14. Wood of Melia indica, Anantapur, Paris Exhibition 1900 15. Wood of Cedrela toona, Nilgiris, Paris Exhibition 1900 16. Wood of Premna bengalensis, Assam, Paris Exhibition 1900 17. Wood of Artocarpus chaplasha, Assam, Paris Exhibition 1900 18. Wood of Artocarpus integrifolia, Nilgiris, Paris Exhibition 1900 19. Wood of Ulmus wallichiana, N. India, Paris Exhibition 1900 20. Wood of Diospyros kurzii , India, Paris Exhibition 1900 21. Wood of Hardwickia binata, Kistna, Paris Exhibition 1900 22. Flowers of Heterotheca inuloides, Mexico, Paris Exhibition 1900 23. Leaves of Datura Stramonium, Paris Exhibition 1900 24. Plant of Mentha viridis, Paris Exhibition 1900 25. Plant of Monsonia ovata, S. -

Chile: a Journey to the End of the World in Search of Temperate Rainforest Giants
Eliot Barden Kew Diploma Course 53 July 2017 Chile: A Journey to the end of the world in search of Temperate Rainforest Giants Valdivian Rainforest at Alerce Andino Author May 2017 1 Eliot Barden Kew Diploma Course 53 July 2017 Table of Contents 1. Title Page 2. Contents 3. Table of Figures/Introduction 4. Introduction Continued 5. Introduction Continued 6. Aims 7. Aims Continued / Itinerary 8. Itinerary Continued / Objective / the Santiago Metropolitan Park 9. The Santiago Metropolitan Park Continued 10. The Santiago Metropolitan Park Continued 11. Jardín Botánico Chagual / Jardin Botanico Nacional, Viña del Mar 12. Jardin Botanico Nacional Viña del Mar Continued 13. Jardin Botanico Nacional Viña del Mar Continued 14. Jardin Botanico Nacional Viña del Mar Continued / La Campana National Park 15. La Campana National Park Continued / Huilo Huilo Biological Reserve Valdivian Temperate Rainforest 16. Huilo Huilo Biological Reserve Valdivian Temperate Rainforest Continued 17. Huilo Huilo Biological Reserve Valdivian Temperate Rainforest Continued 18. Huilo Huilo Biological Reserve Valdivian Temperate Rainforest Continued / Volcano Osorno 19. Volcano Osorno Continued / Vicente Perez Rosales National Park 20. Vicente Perez Rosales National Park Continued / Alerce Andino National Park 21. Alerce Andino National Park Continued 22. Francisco Coloane Marine Park 23. Francisco Coloane Marine Park Continued 24. Francisco Coloane Marine Park Continued / Outcomes 25. Expenditure / Thank you 2 Eliot Barden Kew Diploma Course 53 July 2017 Table of Figures Figure 1.) Valdivian Temperate Rainforest Alerce Andino [Photograph; Author] May (2017) Figure 2. Map of National parks of Chile Figure 3. Map of Chile Figure 4. Santiago Metropolitan Park [Photograph; Author] May (2017) Figure 5. -

Bosque Pehuén Park's Flora: a Contribution to the Knowledge of the Andean Montane Forests in the Araucanía Region, Chile Author(S): Daniela Mellado-Mansilla, Iván A
Bosque Pehuén Park's Flora: A Contribution to the Knowledge of the Andean Montane Forests in the Araucanía Region, Chile Author(s): Daniela Mellado-Mansilla, Iván A. Díaz, Javier Godoy-Güinao, Gabriel Ortega-Solís and Ricardo Moreno-Gonzalez Source: Natural Areas Journal, 38(4):298-311. Published By: Natural Areas Association https://doi.org/10.3375/043.038.0410 URL: http://www.bioone.org/doi/full/10.3375/043.038.0410 BioOne (www.bioone.org) is a nonprofit, online aggregation of core research in the biological, ecological, and environmental sciences. BioOne provides a sustainable online platform for over 170 journals and books published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Web site, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/page/terms_of_use. Usage of BioOne content is strictly limited to personal, educational, and non-commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. R E S E A R C H A R T I C L E ABSTRACT: In Chile, most protected areas are located in the southern Andes, in mountainous land- scapes at mid or high altitudes. Despite the increasing proportion of protected areas, few have detailed inventories of their biodiversity. This information is essential to define threats and develop long-term • integrated conservation programs to face the effects of global change. -

Preliminary Classification of Leotiomycetes
Mycosphere 10(1): 310–489 (2019) www.mycosphere.org ISSN 2077 7019 Article Doi 10.5943/mycosphere/10/1/7 Preliminary classification of Leotiomycetes Ekanayaka AH1,2, Hyde KD1,2, Gentekaki E2,3, McKenzie EHC4, Zhao Q1,*, Bulgakov TS5, Camporesi E6,7 1Key Laboratory for Plant Diversity and Biogeography of East Asia, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, Yunnan, China 2Center of Excellence in Fungal Research, Mae Fah Luang University, Chiang Rai, 57100, Thailand 3School of Science, Mae Fah Luang University, Chiang Rai, 57100, Thailand 4Landcare Research Manaaki Whenua, Private Bag 92170, Auckland, New Zealand 5Russian Research Institute of Floriculture and Subtropical Crops, 2/28 Yana Fabritsiusa Street, Sochi 354002, Krasnodar region, Russia 6A.M.B. Gruppo Micologico Forlivese “Antonio Cicognani”, Via Roma 18, Forlì, Italy. 7A.M.B. Circolo Micologico “Giovanni Carini”, C.P. 314 Brescia, Italy. Ekanayaka AH, Hyde KD, Gentekaki E, McKenzie EHC, Zhao Q, Bulgakov TS, Camporesi E 2019 – Preliminary classification of Leotiomycetes. Mycosphere 10(1), 310–489, Doi 10.5943/mycosphere/10/1/7 Abstract Leotiomycetes is regarded as the inoperculate class of discomycetes within the phylum Ascomycota. Taxa are mainly characterized by asci with a simple pore blueing in Melzer’s reagent, although some taxa have lost this character. The monophyly of this class has been verified in several recent molecular studies. However, circumscription of the orders, families and generic level delimitation are still unsettled. This paper provides a modified backbone tree for the class Leotiomycetes based on phylogenetic analysis of combined ITS, LSU, SSU, TEF, and RPB2 loci. In the phylogenetic analysis, Leotiomycetes separates into 19 clades, which can be recognized as orders and order-level clades.