Diversity and Co-Occurrence Patterns of Microbial Communities in the Fertilized Eggs of Thitarodes Insect, the Implication on the Occurrence of Chinese Cordyceps
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chapter 11 Marine Fungi Associated with Antarctic Macroalgae
Chapter 11 Marine Fungi Associated with Antarctic Macroalgae Mayara B. Ogaki, Maria T. de Paula, Daniele Ruas, Franciane M. Pellizzari, César X. García-Laviña, and Luiz H. Rosa Abstract Fungi are well known for their important roles in terrestrial ecosystems, but filamentous and yeast forms are also active components of microbial communi- ties from marine ecosystems. Marine fungi are particularly abundant and relevant in coastal systems where they can be found in association with large organic substrata, like seaweeds. Antarctica is a rather unexplored region of the planet that is being influenced by strong and rapid climate change. In the past decade, several efforts have been made to get a thorough inventory of marine fungi from different environ- ments, with a particular emphasis on those associated with the large communities of seaweeds that abound in littoral and infralittoral ecosystems. The algicolous fungal communities obtained were characterized by a few dominant species and a large number of singletons, as well as a balance among endemic, indigenous, and cold- adapted cosmopolitan species. The long-term monitoring of this balance and the dynamics of richness, dominance, and distributional patterns of these algicolous fungal communities is proposed to understand and model the influence of climate change on the maritime Antarctic biota. In addition, several fungal isolates from marine Antarctic environments have shown great potential as producers of bioactive natural products and enzymes and may represent attractive sources of biotechno- logical products. M. B. Ogaki · M. T. de Paula · D. Ruas · L. H. Rosa (*) Departamento de Microbiologia, Universidade Federal de Minas Gerais, Belo Horizonte, MG, Brazil e-mail: [email protected] F. -

Cordyceps Medicinal Fungus: Harvest and Use in Tibet
HerbalGram 83 • August – October 2009 83 • August HerbalGram Kew’s 250th Anniversary • Reviving Graeco-Arabic Medicine • St. John’s Wort and Birth Control The Journal of the American Botanical Council Number 83 | August – October 2009 Kew’s 250th Anniversary • Reviving Graeco-Arabic Medicine • Lemongrass for Oral Thrush • Hibiscus for Blood Pressure • St. John’s Wort and BirthWort Control • St. John’s Blood Pressure • HibiscusThrush for Oral for 250th Anniversary Medicine • Reviving Graeco-Arabic • Lemongrass Kew’s US/CAN $6.95 Cordyceps Medicinal Fungus: www.herbalgram.org Harvest and Use in Tibet www.herbalgram.org www.herbalgram.org 2009 HerbalGram 83 | 1 STILL HERBAL AFTER ALL THESE YEARS Celebrating 30 Years of Supporting America’s Health The year 2009 marks Herb Pharm’s 30th anniversary as a leading producer and distributor of therapeutic herbal extracts. During this time we have continually emphasized the importance of using the best quality certified organically cultivated and sustainably-wildcrafted herbs to produce our herbal healthcare products. This is why we created the “Pharm Farm” – our certified organic herb farm, and the “Plant Plant” – our modern, FDA-audited production facility. It is here that we integrate the centuries-old, time-proven knowledge and wisdom of traditional herbal medicine with the herbal sciences and technology of the 21st Century. Equally important, Herb Pharm has taken a leadership role in social and environmental responsibility through projects like our use of the Blue Sky renewable energy program, our farm’s streams and Supporting America’s Health creeks conservation program, and the Botanical Sanctuary program Since 1979 whereby we research and develop practical methods for the conser- vation and organic cultivation of endangered wild medicinal herbs. -

Tesis (4.391Mb)
UNIVERSIDAD AUTÓNOMA DEL ESTADO DE MORELOS INSTITUTO DE INVESTIGACIÓN EN CIENCIAS BÁSICAS Y APLICADAS CENTRO DE INVESTIGACIÓN EN DINÁMICA CELULAR Análisis morfológico y molecular de Aspergillus sydowii en condiciones de baja actividad de agua. T E S I S QUE PARA OBTENER EL GRADO DE MAESTRO EN CIENCIAS PRESENTA: Lic. Gisell Valdés Muñoz DIRECTOR DE TESIS Dr. Ramón Alberto Batista García Sinodales Cuernavaca, Morelos Enero, 2021 Sinodales 1 Sinodales Dr. Carlos Eliud Angulo Valadez Dra. Sonia Dávila Ramos Dr. Raúl Peralta Rodríguez Dra. María del Rayo Sánchez Carbente Dr. Ramón Alberto Batista García 2 3 4 Agradecimientos Estos dos años de maestría en México han sido de los mejores de mi vida, he aprendido tantas cosas nuevas y he superado muchos retos incluso miedos, y hay tantas personas a las cuales agradecer: A mi tutor Dr. Ramón A. Batista García, por su aceptación, guía, palabras fuertes y cariñosas, y por toda su destreza para elaborar un camino bonito de estudios y superación para mí. A mi asesora y amiga MsC. Irina Jiménez Gómez, por cada día enseñarme algo nuevo, por enseñarme a trabajar en el laboratorio con los hongos, por nuestras experiencias microscópicas en Ensenada y porque verdaderamente con ella mi maestría fue excelente. A la Dra. María del Rayo Sánchez Carbente y Dra. Sonia Dávila Ramos porque en cada examen semestral me apoyaron a mejorar esta investigación. Al Dr. Ayixon Sánchez Reyes, MsC. Hugo Castelán y MsC. Yordanis Pérez Llano por su ayuda bioinformática tan amable y desinteresada, gracias por compartir esos conocimientos. A las chicas ―Aspergillus‖ Debu, Heidy, Lyselle, Adri por nuestros estudios en conjunto, por darnos apoyo y siempre estar disponibles, gracias por su amistad. -

Lists of Names in Aspergillus and Teleomorphs As Proposed by Pitt and Taylor, Mycologia, 106: 1051-1062, 2014 (Doi: 10.3852/14-0
Lists of names in Aspergillus and teleomorphs as proposed by Pitt and Taylor, Mycologia, 106: 1051-1062, 2014 (doi: 10.3852/14-060), based on retypification of Aspergillus with A. niger as type species John I. Pitt and John W. Taylor, CSIRO Food and Nutrition, North Ryde, NSW 2113, Australia and Dept of Plant and Microbial Biology, University of California, Berkeley, CA 94720-3102, USA Preamble The lists below set out the nomenclature of Aspergillus and its teleomorphs as they would become on acceptance of a proposal published by Pitt and Taylor (2014) to change the type species of Aspergillus from A. glaucus to A. niger. The central points of the proposal by Pitt and Taylor (2014) are that retypification of Aspergillus on A. niger will make the classification of fungi with Aspergillus anamorphs: i) reflect the great phenotypic diversity in sexual morphology, physiology and ecology of the clades whose species have Aspergillus anamorphs; ii) respect the phylogenetic relationship of these clades to each other and to Penicillium; and iii) preserve the name Aspergillus for the clade that contains the greatest number of economically important species. Specifically, of the 11 teleomorph genera associated with Aspergillus anamorphs, the proposal of Pitt and Taylor (2014) maintains the three major teleomorph genera – Eurotium, Neosartorya and Emericella – together with Chaetosartorya, Hemicarpenteles, Sclerocleista and Warcupiella. Aspergillus is maintained for the important species used industrially and for manufacture of fermented foods, together with all species producing major mycotoxins. The teleomorph genera Fennellia, Petromyces, Neocarpenteles and Neopetromyces are synonymised with Aspergillus. The lists below are based on the List of “Names in Current Use” developed by Pitt and Samson (1993) and those listed in MycoBank (www.MycoBank.org), plus extensive scrutiny of papers publishing new species of Aspergillus and associated teleomorph genera as collected in Index of Fungi (1992-2104). -

Drevoznehodnocujúce Huby 2018
LESNÍCKA FAKULTA TU vo Zvolene Katedra integrovanej ochrany lesa a krajiny DREVÁRSKA FAKULTA TU vo Zvolene Katedra mechanickej technológie dreva FAKULTA EKOLÓGIE A ENVIRONMENTALISTIKY TU vo Zvolene Katedra biológie a všeobecnej ekológie FAKULTA PRÍRODNÝCH VIED UMB V BANSKEJ BYSTRICI Katedra biológie a ekológie DREVOZNEHODNOCUJÚCE HUBY 2018 Vedecký recenzovaný zborník vydaný pri príležitosti životného jubilea prof. Ing. Ladislava Reinprechta, CSc. a prof. RNDr. Jána Gápera, CSc. 2018 1 LESNÍCKA FAKULTA TU vo Zvolene Katedra integrovanej ochrany lesa a krajiny DREVÁRSKA FAKULTA TU vo Zvolene Katedra mechanickej technológie dreva FAKULTA EKOLÓGIE A ENVIRONMENTALISTIKY TU vo Zvolene Katedra biológie a všeobecnej ekológie FAKULTA PRÍRODNÝCH VIED UMB V BANSKEJ BYSTRICI Katedra biológie a ekológie DREVOZNEHODNOCUJÚCE HUBY 2018 Vedecký recenzovaný zborník vydaný pri príležitosti životného jubilea prof. Ing. Ladislava Reinprechta, CSc. a prof. RNDr. Jána Gápera, CSc. 2018 2 DREVOZNEHODNOCUJÚCE HUBY 2018 Vedecký recenzovaný zborník vydaný pri príležitosti životného jubilea prof. Ing. Ladislava Reinprechta, CSc. a prof. RNDr. Jána Gápera, CSc. Hronská 6 Hlinícka 2 Nám. SNP 8 974 01 Banská Bystrica 831 52 Bratislava 975 66 Banská Bystrica www.laboratornepristroje.sk www.optoteam.sk www.lesy.sk Recenzenti : Ing. Andrej Kunca, PhD. Ing. Ľuboš Blaško, PhD. Ing. Erik Nosáľ, PhD. Ing. Stanislav Jochim, PhD. Editori: Zuzana Vidholdová, Pavol Hlaváč Rozsah: 167 strán Vydanie: I. 2018 Náklad: 100 kusov na CD Tlač – výroba CD: Afinita, s.r.o. Sliač Vydavateľ: Technická univerzita vo Zvolene Všetky príspevky publikované v zborníku boli recenzované anonymnou formou vyššie uvedenými recenzentmi z oblasti vysokého školstva, vedy a odbornej praxe. Za obsah príspevkov zodpovedajú autori a recenzenti. Rukopis neprešiel jazykovou úpravou. -

Characterization and Phylogenetic Analysis of the Complete Mitochondrial Genome of the Medicinal Fungus Laetiporus Sulphureus
www.nature.com/scientificreports OPEN Characterization and phylogenetic analysis of the complete mitochondrial genome of the Received: 29 December 2017 Accepted: 24 May 2018 medicinal fungus Laetiporus Published: xx xx xxxx sulphureus Qiang Li1,3, Mei Yang2, Cheng Chen4, Chuan Xiong1, Xin Jin1, Zhigang Pu1,5 & Wenli Huang1,5 The medicinal fungus Laetiporus sulphureus is widely distributed worldwide. To screen for molecular markers potentially useful for phylogenetic analyses of this species and related species, the mitochondrial genome of L. sulphureus was sequenced and assembled. The complete circular mitochondrial genome was 101,111 bp long, and contained 38 protein-coding genes (PCGs), 2 rRNA genes, and 25 tRNA genes. Our BLAST search aligned about 6.1 kb between the mitochondrial and nuclear genomes of L. sulphureus, indicative of possible gene transfer events. Both the GC and AT skews in the L. sulphureus mitogenome were negative, in contrast to the other seven Polyporales species tested. Of the 15 PCGs conserved across the seven species of Polyporales, the lengths of 11 were unique in the L. sulphureus mitogenome. The Ka/Ks of these 15 PCGs were all less than 1, indicating that PCGs were subject to purifying selection. Our phylogenetic analysis showed that three single genes (cox1, cob, and rnl) were potentially useful as molecular markers. This study is the frst publication of a mitochondrial genome in the family Laetiporaceae, and will facilitate the study of population genetics and evolution in L. sulphureus and other species in this family. Te fruiting body of Laetiporus sulphureus (Bull.) Murill, 1904 (Basidiomycota: Polyporales), with its striking citrus-yellow to pale orange color, is considered a cosmopolitan species, distributed from the boreal to tropical climactic zones1. -

Abstract a Geographic Analysis of The
ABSTRACT A GEOGRAPHIC ANALYSIS OF THE VULNERABILITIES AND COPING STRATEGIES OF TIBETAN HERDERS IN GANSU, CHINA by Luci Xi Lu A dominant narrative of rangeland degradation in western China is that degradation is caused by overstocking and poor land use practices. Consequently, the state has designed and implemented a series of grassland policies (e.g., privatizing common grazing land, depopulating livestock, and relocating herders) in pastoral regions of China. Although the government sees communal rangeland management as inefficient and unsustainable, collective rangeland management persists. Using Machu County in Gansu Province as a case study, I examined the differences between de jure and de facto land tenure on eastern Tibetan Plateau. This study employed semi-structured interviews and extensive participant observation with 43 Amdo Tibetan herders in Machu County, Gansu province, Western China. I also triangulated the first-hand empirical data with the secondary data I obtained from Bureau of Poverty Alleviation and Bureau of Animal Husbandry in Machu. Research findings show that instead of herding individually and maximizing the economic benefit, the majority of herders are pooling resources communally in kin-based encampments in order to avoid risks. Because of the spatio- temporal variation of precipitation, certain encampments perceive themselves more vulnerable to water shortage and topography-related hazards. Renting pastures and seeking alternative livelihoods then become the key strategies for herders to restore mobility and -

Temperature Dependent Lipase Production from Cold and Ph Tolerant Species of Penicillium
Mycosphere (2016) www.mycosphere.org ISSN 2077 7019 Article Doi 10.5943/mycosphere/si/3b/5 Copyright © Guizhou Academy of Agricultural Sciences Temperature dependent lipase production from cold and pH tolerant species of Penicillium Pandey N1, Dhakar K1, Jain R1, Pandey A1 1Biotechnological Applications,G. B. Pant National Institute of Himalayan Environment and Sustainable Development, Kosi-Katarmal, Almora - 263 643, Uttarakhand, India Pandey N, Dhakar K, Jain R, Pandey A 2016 – Temperature dependent lipase production from cold and pH tolerant species of Penicillium. Mycosphere (special issue), Doi 10.5943/mycosphere/si/3b/5 Abstract The psychrotolerant microorganisms are receiving attention of the scientific community due to their ability to produce biotechnological products. The present study is focused on the diversity of cold and pH tolerant isolates of Penicillium spp with respect to their potential to produce cold active lipases. The characterization of the fungal isolates was done using polyphasic approach (morphological and molecular methods). The isolates were found to have tolerance for temperature from 4-35 ºC (opt.21-25 ºC) and pH 2-14 (opt. 5-7). Lipase production was investigated under the influence of temperature between 5-35 ºC. The fungal isolates were found to produce lipase, optimally at different temperatures,up to 25 days of incubation. Maximum lipase production was recorded at 15 and 25 ºC temperatures, whereas it was minimum at 5 and 35 ºC. Three fungal isolates, designated as GBPI_P98, GBPI_P150 and GBPI_P228, were found to produce optimal lipase at 25 ºC whereas seven isolates, GBPI_P8, GBPI_P36, GBPI_P72, GBPI_P101, GBPI_P141, GBPI_P188 and GBPI_P222, showed maximum lipase prodution at 15 ºC. -

Utilisation Des Lepidopteres En Medecine Traditionnelle Et Moderne
Université de Lille Faculté de Pharmacie de Lille Année Universitaire 2019/2020 THESE POUR LE DIPLOME D'ETAT DE DOCTEUR EN PHARMACIE Soutenue publiquement le 20 mars 2020 Par M. Bracq Tristan _____________________________ UTILISATION DES LEPIDOPTERES EN MEDECINE TRADITIONNELLE ET MODERNE _____________________________ Membres du jury : Président et conseiller de thèse : Docteur Vincent ROUMY, Maître de Conférences, Faculté des Sciences Pharmaceutiques et Biologiques de Lille, laboratoire de Pharmacognosie Assesseur : Docteur Philippe GERVOIS, Maître de Conférences, HDR, Faculté des Sciences Pharmaceutiques et Biologiques de Lille, laboratoire de Biochimie Membre extérieur : Docteur Dominique ANGENAULT, Pharmacien d’officine à Bully- les-mines 1 2 Université de Lille Faculté de Pharmacie de Lille Année Universitaire 2019/2020 THESE POUR LE DIPLOME D'ETAT DE DOCTEUR EN PHARMACIE Soutenue publiquement le 20 mars 2020 Par M. Bracq Tristan _____________________________ UTILISATION DES LEPIDOPTERES EN MEDECINE TRADITIONNELLE ET MODERNE _____________________________ Membres du jury : Président et conseiller de thèse : Docteur Vincent ROUMY, Maître de Conférences, Faculté des Sciences Pharmaceutiques et Biologiques de Lille, laboratoire de Pharmacognosie Assesseur : Docteur Philippe GERVOIS, Maître de Conférences, HDR, Faculté des Sciences Pharmaceutiques et Biologiques de Lille, laboratoire de Biochimie Membre extérieur : Docteur Dominique ANGENAULT, Pharmacien d’officine à Bully- les-mines 3 Faculté de Pharmacie de Lille 3, rue du Professeur -

A New Species of Pharmacis Hübner, 1820 from Spain with a Brief Review of the Genera Pharmacis and Korscheltellus Börner, 1920 (Lepidoptera, Hepialidae)
Nota Lepi. 41(2) 2018: 225–249 | DOI 10.3897/nl.41.26835 A new species of Pharmacis Hübner, 1820 from Spain with a brief review of the genera Pharmacis and Korscheltellus Börner, 1920 (Lepidoptera, Hepialidae) Axel Kallies1, Teresa Farino2 1 University of Melbourne, School of BioSciences, Parkville, 3010 Victoria, Australia; [email protected] 2 Apartado de Correos 59, 39570 Potes, Cantabria, Spain; [email protected] http://zoobank.org/B506D8D1-960D-4267-9140-2B1D8A11F449 Received 25 May 2018; accepted 21 September 2018; published: 9 November 2018 Subject Editor: Maria Heikkilä. Abstract. We here describe a new ghost moth (Hepialidae) species, Pharmacis cantabricus sp. n. from the Picos de Europa National Park, Cantabria, in northern Spain. The new species belongs to a group of mostly day-flying species that are restricted to the European Alps and some mountain ranges of southern Europe. Based on morphology and analysis of mitochondrial COI gene sequences, the new species is closely related to Pharmacis aemilianus (Constantini, 1911), an endemic of the Italian Apennines. However, Pharmacis cantabricus sp. n. can easily be distinguished from all related species based on both external and genitalic characters. We briefly review and illustrate all species of the genus Pharmacis Hübner, 1820 and discuss its relationship with the related genus Korscheltellus Börner, 1920. We reinstate Hepialus castillanus Oberthür, 1883 as a distinct species and transfer it to Korscheltellus (stat. rev., comb. n.). Resumen. Describimos una nueva especie de Hepialidae, Pharmacis cantabricus sp. n. del Parque Nacional de Picos de Europa, Cantabria, España septentrional. La nueva especie pertenece a un grupo de especies de vuelo diurno cuya distribución se limita a los Alpes europeos y otras cadenas montañosas de Europa merid- ional. -

A New Species of Thitarodes Viette (Lepidoptera, Hepialidae) from Japan
Bull. Kitakyushu Mus. Nat. Hist., 15: 35-41, pi. II. March 28, 1996 A New Species of Thitarodes Viette (Lepidoptera, Hepialidae) from Japan Kyoichiro Ueda Kitakyushu Museum and Institute of Natural History, Nishihonmachi 3, Yahatahigashiku, Kitakyushu 805,Japan (Received November 16, 1995) Abstract A new species of Thitarodes Viette is named and described: Thitarodes nipponensis. Its morphology is described and figured. The genus Thitarodes was erected by Viette (1968) to accommodate small, dark species ofHepialidae distinguished by thepresence ofan acute process from the base ofthe valva; Hepialus armoricanus Oberthur, 1909 was designated as the type-species. He added three new species from Nepal: Thitarodes danieli, T. eberti and T. dierli to this genus (Viette, 1. c). Chu & Wang (1985) revised hepialid specimens from China associated with the "insect-herb" (the fungus Cordyceps) and described 19 species in five genera. They did not adopt the genus Thitarodes Viette in their system as they regarded the morphological difference in male genitalia insignificant as a generic character and that using Viette's criteria would lead to the establishment of many new genera. They assigned 13 species to the genus Hepialus Fabricius. They, however, described many character differences in the labial palpus and even wing venation among those 13 species, the differences of which are usually regarded as generic characters. If we followed Viette's definition ofthegenus Thitarodes, at least four species might be assigned to the genus: kangdingensis, oblifurcus, kangdingroides, and zhangmoensis. In this paper I describe onenew species ofthegenus Thitarodes, the first record of the genus from Japan. The terminology used in descriptions of male and female genitalia follows mainly Ueda (1988). -
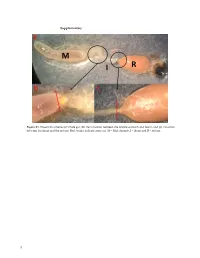
Supplementary File 1
Supplementary Figure S1. Dissection scheme (a) whole gut, (b) the transition between the middle stomach and ileum, and (c) transition between the ileum and the rectum. Red streaks indicate areas cut. M = Mid stomach, I = ileum and R = rectum. 1 Figure S2. Relative abundance of the 20 most abundant fungal OTUs across months per gut part. Taxonomic assignments are provided in Table S1. 2 Figure S3. Relative abundance of the bacterial OUTs (with abundance > 1%) across months per gut part. The color code is given at the genus level. 3 Table S1. Taxonomic assignments of fungal OTUs by Blast. OTU# Taxonomy by blast nt collection 11/11‐2020 Phylum Accesion# OTU_1 Aureobasidium pullulans Ascomycota MW085051 OTU_2 Meyerozyma guilliermondii Ascomycota MT988167 OTU_3 Starmerella apicola Ascomycota KY101940 OTU_4 Debaryomyces hansenii Ascomycota MW051606 OTU_5 Engyodontium sp. Ascomycota MN905797 Basidiomy‐ OTU_6 Mrakia sp. MT505696 cota Basidiomy‐ OTU_7 Tausonia pullulans MN900123 cota OTU_8 Unknown OTU_9 Unknown OTU_10 Penicillium corylophilum Ascomycota MT906500 OTU_11 Penicillium sp. Ascomycota MT993349 OTU_12 Cladosporium sp. Ascomycota MW077705 Phaffia rhodozyma/Xanthophyllomyces dendro‐ Basidiomy‐ OTU_13 KY104501/DQ904243 rhous cota Basidiomy‐ OTU_14 Filobasidium wieringae MN899199 cota OTU_15 Hanseniaspora uvarum Ascomycota MN556596 Basidiomy‐ OTU_16 Mrakia gelida MN460370 cota OTU_17 Uncultured fungus clone MK717974 OTU_18 Cladosporium allicinum Ascomycota MT974153 OTU_20 Taphrina carpini Ascomycota MK782181 Basidiomy‐ OTU_25 Papiliotrema