Phenotypic Diversity in the Wheat Wild Relative Aegilops Longissima
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Pdf, 366.38 Kb
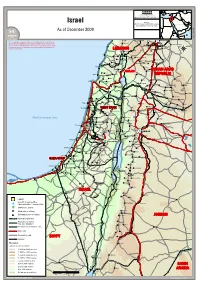
FF II CC SS SS Field Information and Coordination Support Section Division of Operational Services Israel Sources: UNHCR, Global Insight digital mapping © 1998 Europa Technologies Ltd. As of December 2009 Israel_Atlas_A3PC.WOR Dahr al Ahmar Jarba The designations employed and the presentation of material on this map do not imply the expression of any opinion whatsoever on the part of the 'Aramtah Ma'adamiet Shih Harran al 'Awamid Secretariat of the United Nations concerning the legal status of any country, Qatana Haouch Blass 'Artuz territory, city or area of its authorities or concerning the delimitation of its Najha frontiers or boundaries LEBANON Al Kiswah Che'baâ Douaïr Al Khiyam Metulla Sa`sa` ((( Kafr Dunin Misgav 'Am Jubbata al Khashab ((( Qiryat Shemons Chakra Khan ar Rinbah Ghabaqhib Rshaf Timarus Bent Jbail((( Al Qunaytirah Djébab Nahariyya El Harra ((( Dalton An Namir SYRIAN ARAB Jacem Hatzor GOLANGOLAN Abu-Senan GOLANGOLAN Ar Rama Acre ((( Boutaiha REPUBLIC Bi'nah Sahrin Tamra Shahba Tasil Ash Shaykh Miskin ((( Kefar Hittim Bet Haifa ((( ((( ((( Qiryat Motzkin ((( ((( Ibta' Lavi Ash Shajarah Dâail Kafr Kanna As Suwayda Ramah Kafar Kama Husifa Ath Tha'lah((( ((( ((( Masada Al Yadudah Oumm Oualad ((( ((( Saïda 'Afula ((( ((( Dar'a Al Harisah ((( El 'Azziya Irbid ((( Al Qrayyah Pardes Hanna Besan Salkhad ((( ((( ((( Ya'bad ((( Janin Hadera ((( Dibbin Gharbiya El-Ne'aime Tisiyah Imtan Hogla Al Manshiyah ((( ((( Kefar Monash El Aânata Netanya ((( WESTWEST BANKBANK WESTWEST BANKBANKTubas 'Anjara Khirbat ash Shawahid Al Qar'a' -

Sefer Haner on Mesechet Bava Kamma,Benefits
Sefer HaNer on Mesechet Bava Kamma Sefer HaNer on Mesechet Bava Kamma: A Review by:Rabbi Yosaif Mordechai Dubovick Not every important work written by a Rishon is blessed with popularity.[1] While many texts were available throughout the generations and utilized to their utmost; others were relegated to obscurity, being published as recently as this century, or even this year. Nearly a month doesn't pass without a "new" Rishon being made available to the public, and often enough in a critical edition. While each work must be evaluated on its own merit, as a whole, every commentary, every volume of Halachic rulings adds to our knowledge and Torah study.[2] From the Geonic era through the Rishonim, North Africa was blessed with flourishing Torah centers, Kairouan in Tunisia (800-1057),[3] Fostat (Old Cairo) in Egypt, and many smaller cities as well. Perhaps the crown jewel of "pre-Rambam" Torah study was the sefer Hilchot Alfasi by R' Yitchock Alfasi (the Rif).[4] Many Rishonim focused their novella around the study of Rif,[5] the Rambam taught Rif in lieu of Talmud,[6] and a pseudo-Rashi and Tosefot were developed to encompass the texts used and accompany its study.[7] In Aghmat, a little known city in Morocco, circa the Rambam's lifetime, rose up a little known Chacham whose work is invaluable in studying Rif, and by correlation, the Talmud Bavli as a whole. Yet, this Chacham was unheard of, for the most part, until the past half century. R' Zechariya b. Yehuda of Aghmat, authored a compendium of Geonim, Rishonim, and personal exegesis on Rif. -

Ben-Gurion University of the Negev the Jacob Blaustein Institutes for Desert Research the Albert Katz International School for Desert Studies
Ben-Gurion University of the Negev The Jacob Blaustein Institutes for Desert Research The Albert Katz International School for Desert Studies Evolution of settlement typologies in rural Israel Thesis submitted in partial fulfillment of the requirements for the degree of "Master of Science" By: Keren Shalev November, 2016 “Human settlements are a product of their community. They are the most truthful expression of a community’s structure, its expectations, dreams and achievements. A settlement is but a symbol of the community and the essence of its creation. ”D. Bar Or” ~ III ~ תקציר למשבר הדיור בישראל השלכות מרחיקות לכת הן על המרחב העירוני והן על המרחב הכפרי אשר עובר תהליכי עיור מואצים בעשורים האחרונים. ישובים כפריים כגון קיבוצים ומושבים אשר התבססו בעבר בעיקר על חקלאות, מאבדים מאופיים הכפרי ומייחודם המקורי ומקבלים צביון עירוני יותר. נופי המרחב הכפרי הישראלי נעלמים ומפנים מקום לשכונות הרחבה פרבריות סמי- עירוניות, בעוד זהותה ודמותה הייחודית של ישראל הכפרית משתנה ללא היכר. תופעת העיור המואץ משפיעה לא רק על נופים כפריים, אלא במידה רבה גם על מרחבים עירוניים המפתחים שכונות פרבריות עם בתים צמודי קרקע על מנת להתחרות בכוח המשיכה של ישובים כפריים ולמשוך משפחות צעירות חזקות. כתוצאה מכך, סובלים המרחבים העירוניים, הסמי עירוניים והכפריים מאובדן המבנה והזהות המקוריים שלהם והשוני ביניהם הולך ומיטשטש. על אף שהנושא מעלה לא מעט סוגיות תכנוניות חשובות ונחקר רבות בעולם, מעט מאד מחקר נעשה בנושא בישראל. מחקר מקומי אשר בוחן את תהליכי העיור של המרחב הכפרי דרך ההיסטוריה והתרבות המקומית ולוקח בחשבון את התנאים המקומיים המשתנים, מאפשר התבוננות ואבחנה מדויקים יותר על ההשלכות מרחיקות הלכת. על מנת להתגבר על הבסיס המחקרי הדל בנושא, המחקר הנוכחי החל בבניית בסיס נתונים רחב של 84 ישובים כפריים (קיבוצים, מושבים וישובים קהילתיים( ומצייר תמונה כללית על תהליכי העיור של המרחב הכפרי ומאפייניה. -

The Humanitarian Monitor CAP Occupied Palestinian Territory Number 17 September 2007
The Humanitarian Monitor CAP occupied Palestinian territory Number 17 September 2007 Overview- Key Issues Table of Contents Update on Continued Closure of Gaza Key Issues 1 - 2 Crossings Regional Focus 3 Access and Crossings Rafah and Karni crossings remain closed after more than threemonths. Protection of Civilians 4 - 5 The movement of goods via Gaza border crossings significantly Child Protection 6-7 declined in September compared to previous months. The average Violence & Private 8-9 of 106 truckloads per day that was recorded between 19 June and Property 13 September has dropped to approximately 50 truckloads per day 10 - 11 since mid-September. Sufa crossing (usually opened 5 days a week) Access was closed for 16 days in September, including 8 days for Israeli Socio-economic 12 - 13 holidays, while Kerem Shalom was open only 14 days throughout Conditions the month. The Israeli Civil Liaison Administration reported that the Health 14 - 15 reduction of working hours was due to the Muslim holy month Food Security & 16 - 18 of Ramadan, Jewish holidays and more importantly attacks on the Agriculture crossings by Palestinian militants from inside Gaza. Water & Sanitation 19 Impact of Closure Education 20 As a result of the increased restrictions on Gaza border crossings, The Response 21 - 22 an increasing number of food items – including fruits, fresh meat and fish, frozen meat, frozen vegetables, chicken, powdered milk, dairy Sources & End Notes 23 - 26 products, beverages and cooking oil – are experiencing shortages on the local market. The World Food Programme (WFP) has also reported significant increases in the costs of these items, due to supply, paid for by deductions from overdue Palestinian tax increases in prices on the global market as well as due to restrictions revenues that Israel withholds. -

Israel: Growing Pains at 60
Viewpoints Special Edition Israel: Growing Pains at 60 The Middle East Institute Washington, DC Middle East Institute The mission of the Middle East Institute is to promote knowledge of the Middle East in Amer- ica and strengthen understanding of the United States by the people and governments of the region. For more than 60 years, MEI has dealt with the momentous events in the Middle East — from the birth of the state of Israel to the invasion of Iraq. Today, MEI is a foremost authority on contemporary Middle East issues. It pro- vides a vital forum for honest and open debate that attracts politicians, scholars, government officials, and policy experts from the US, Asia, Europe, and the Middle East. MEI enjoys wide access to political and business leaders in countries throughout the region. Along with information exchanges, facilities for research, objective analysis, and thoughtful commentary, MEI’s programs and publications help counter simplistic notions about the Middle East and America. We are at the forefront of private sector public diplomacy. Viewpoints are another MEI service to audiences interested in learning more about the complexities of issues affecting the Middle East and US rela- tions with the region. To learn more about the Middle East Institute, visit our website at http://www.mideasti.org The maps on pages 96-103 are copyright The Foundation for Middle East Peace. Our thanks to the Foundation for graciously allowing the inclusion of the maps in this publication. Cover photo in the top row, middle is © Tom Spender/IRIN, as is the photo in the bottom row, extreme left. -

Protection of Civilians Weekly Report
U N I TOCHA E D Weekly N A Report: T I O 14N MarchS – 20 March 2007 N A T I O N S| 1 U N I E S OFFICE FOR THE COORDINATION OF HUMANITARIAN AFFAIRS P.O. Box 38712, East Jerusalem, Phone: (+972) 2-582 9962 / 582 5853, Fax: (+972) 2-582 5841 [email protected], www.ochaopt.org Protection of Civilians Weekly Report 14 March – 20 March 2007 Of note this week An UNRWA convoy carrying the Director of UNRWA Operations in Gaza was attacked by a group of armed masked gunmen in the northern Gaza Strip. The convoy escaped unharmed despite numerous shots being fired at the vehicle. Gaza Strip − A Palestinian sniper shot and injured a civilian Israeli utility worker in the Nahal Oz area. The military wing of Hamas claimed responsibility. Nine homemade rockets, one of which detonated inside the Gaza Strip, and two mortar shells were fired by Palestinians throughout the week towards Israel. − Four Israeli military boats opened fire and rounded up 14 Palestinian fishing boats in Rafah and forced them to sail towards deeper waters. IDF vessels tied the boats and ordered the fishermen to jump in the water and swim individually towards the military ships. A total of 54 Palestinian fishermen were interrogated before later being released while two others were arrested. − Seven Palestinians were killed this week as a result of internal violence including an eight year-old girl caught in crossfire during a family dispute. − Eight days have passed since the BBC's reporter was abducted in Gaza City. -
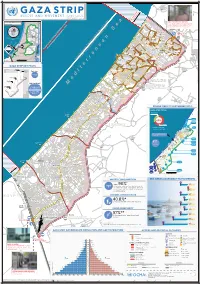
Gaza CRISIS)P H C S Ti P P I U
United Nations Office for the Coordination of Humanitarian Affairs occupied Palestinian territory Zikim e Karmiya s n e o il Z P m A g l in a AGCCESSA ANDZ AMOV EMENTSTRI (GAZA CRISIS)P h c s ti P P i u F a ¥ SEPTEMBER 2014 o nA P N .5 F 1 Yad Mordekhai EREZ CROSSING (BEIT HANOUN) occupied Palestinian territory: ID a As-Siafa OPEN, six days (daytime) a B?week4 for B?3the4 movement d Governorates e e of international workers and limited number of y h s a b R authorized Palestinians including aid workers, medical, P r 2 e A humanitarian cases, businessmen and aid workers. Jenin d 1 e 0 Netiv ha-Asara P c 2 P Tubas r Tulkarm r fo e S P Al Attarta Temporary Wastewater P n b Treatment Lagoons Qalqiliya Nablus Erez Crossing E Ghaboon m Hai Al Amal r Fado's 4 e B? (Beit Hanoun) Salfit t e P P v i Al Qaraya al Badawiya i v P! W e s t R n m (Umm An-Naser) n i o » B a n k a North Gaza º Al Jam'ia ¹¹ M E D I TER RAN EAN Hatabiyya Ramallah da Jericho d L N n r n r KJ S E A ee o Beit Lahia D P o o J g Wastewater Ed t Al Salateen Beit Lahiya h 5 Al Kur'a J a 9 P l D n Treatment Plant D D D D 9 ) D s As Sultan D 1 2 El Khamsa D " Sa D e J D D l i D 0 D s i D D 0 D D d D D m 2 9 Abedl Hamaid D D r D D l D D o s D D a t D D c Jerusalem D D c n P a D D c h D D i t D D s e P! D D A u P 0 D D D e D D D a l m d D D o i t D D l i " D D n . -

This Article Is from the March 2013 Issue Of
This article is from the March 2013 issue of published by The American Phytopathological Society For more information on this and other topics related to plant pathology, we invite you to visit APSnet at www.apsnet.org Identifying New Sources of Resistance to Eyespot of Wheat in Aegilops longissima H. Sheng and T. D. Murray, Department of Plant Pathology, Washington State University, Pullman 99164-6430 Abstract Sheng, H., and Murray, T. D. 2013. Identifying new sources of resistance to eyespot of wheat in Aegilops longissima. Plant Dis. 97:346-353. Eyespot, caused by Oculimacula yallundae and O. acuformis, is an substitution lines containing chromosomes 1Sl, 2Sl, 5Sl, and 7Sl, and a economically important disease of wheat. Currently, two eyespot re- 4Sl7Sl translocation were resistant to O. yallundae. Chromosomes 1Sl, sistance genes, Pch1 and Pch2, are used in wheat breeding programs 2Sl, 4Sl, and 5Sl contributed to resistance to O. acuformis more than but neither provides complete control or prevents yield loss. Aegilops others. Chromosomes 1Sl, 2Sl, 5Sl, and 7Sl provided resistance to both longissima is a distant relative of wheat and proven donor of genes pathogens. This is the first report of eyespot resistance in A. longis- useful for wheat improvement, including disease resistance. Forty A. sima. These results provide evidence that genetic control of eyespot longissima accessions and 83 A. longissima chromosome addition or resistance is present on multiple chromosomes of the Sl genome. This substitution lines were evaluated for resistance to eyespot. Among the research demonstrates that A. longissima is a potential new source of 40 accessions tested, 43% were resistant to O. -

Field Trip 2017 Israel
YEP Field Trip 2017 Israel Technology Tour Itinerary YEP – Young Engineers’ Panel 5.11.2017 DAY 1 • Pickup (08:30 AM): Eilat airport and north beach hotel. • Ramon Crater: The world’s largest erosion crater (makhtesh). A landform unique to Israel, Egypt and Sinai desert, it is a large erosion cirque, created 220 million years ago when oceans covered the area. The Ramon Crater measures 40 km in length and between 2 and 10km in width, shaped like a long heart, and forms Israel’s largest national park, the Ramon Nature Reserve. • Ramon Visitors Center, located on the edge of Makhtesh Ramon is overlooking the Crater. It displays the geography, geology, flora, fauna and history of the region from prehistoric to modern times. A film explains how the Makhtesh was formed and a three-dimensional interactive model helps bring home an understanding of the topography of this unique region. • Sde Boker kibbutz is famous as the home of David Ben Gurion, Israel’s first Prime Minister whose home is now a museum open to the public, and is the feature of a number of supporting exhibits in the kibbutz. Sde Boker is a community founded in 1952 by a number of pioneering families who were later joined by Ben Gurion after an interesting encounter. South of Sde Boker is Ben Gurion’s burial site, which is set in an incredible location overlooking one of the most striking and impressive views in the Negev, across the Zin Valley. 1 • Ashalim (Technology tour) - Solar Energy (thermal and PV) Solar power tower and Solar field. -

The Bedouin Population in the Negev
T The Since the establishment of the State of Israel, the Bedouins h in the Negev have rarely been included in the Israeli public e discourse, even though they comprise around one-fourth B Bedouin e of the Negev’s population. Recently, however, political, d o economic and social changes have raised public awareness u i of this population group, as have the efforts to resolve the n TThehe BBedouinedouin PPopulationopulation status of the unrecognized Bedouin villages in the Negev, P Population o primarily through the Goldberg and Prawer Committees. p u These changing trends have exposed major shortcomings l a in information, facts and figures regarding the Arab- t i iinn tthehe NNegevegev o Bedouins in the Negev. The objective of this publication n The Abraham Fund Initiatives is to fill in this missing information and to portray a i in the n Building a Shared Future for Israel’s comprehensive picture of this population group. t Jewish and Arab Citizens h The first section, written by Arik Rudnitzky, describes e The Abraham Fund Initiatives is a non- the social, demographic and economic characteristics of N Negev profit organization that has been working e Bedouin society in the Negev and compares these to the g since 1989 to promote coexistence and Jewish population and the general Arab population in e equality among Israel’s Jewish and Arab v Israel. citizens. Named for the common ancestor of both Jews and Arabs, The Abraham In the second section, Dr. Thabet Abu Ras discusses social Fund Initiatives advances a cohesive, and demographic attributes in the context of government secure and just Israeli society by policy toward the Bedouin population with respect to promoting policies based on innovative economics, politics, land and settlement, decisive rulings social models, and by conducting large- of the High Court of Justice concerning the Bedouins and scale social change initiatives, advocacy the new political awakening in Bedouin society. -

Autumn 2014 No
Autumn 2014 No. 335 Israel Under Attack WIZO Stands at Israel’s Side Road Safety for Toddlers WIZO Leads the Way There and Back A Story of True Zionism From the Editor Dear Chaverot, to her and the Mexican Jewish community, where there is virtually no assimilation; she also outlines her dreams for Many of the articles in this the future of WIZO Mexico. (page 14) magazine are about people, special people, WIZO people. Miki Doron is a well-known WIZO figure both in Israel and abroad, where he often conducts workshops at conferences. This summer, during Operation How many know that he is a graduate of WIZO Hadassim, Protective Edge, WIZO opened having arrived in Israel alone, as a 15-year-old from Iran. its doors to give shelter and Read Miki’s inspirational story on page 16. peace of mind to hundreds of families from the south of the It’s never too young to learn about road safety. WIZO’s Early country, while our staff and Age Division has a special programme for the toddlers in volunteers all mobilized in our day care centres. (page 18.) different ways. Read all about it on pages 6-11. Many families in Israel cannot afford to give their children Many people talk in despair of the ‘younger’ generation a bar/bat mitzvah celebration. Some of our WIZO branches – many of whom assimilate and have no interest in and in Israel try to provide parties and religious ceremonies for certainly don’t support Israel. But, in this issue we have two needy families in their communities. -

Israel's Rights As a Nation-State in International Diplomacy
Jerusalem Center for Public Affairs Institute for Research and Policy המרכז הירושלמי לענייני ציבור ומדינה )ע"ר( ISRAEl’s RiGHTS as a Nation-State in International Diplomacy Israel’s Rights as a Nation-State in International Diplomacy © 2011 Jerusalem Center for Public Affairs – World Jewish Congress Jerusalem Center for Public Affairs 13 Tel Hai Street, Jerusalem, Israel Tel. 972-2-561-9281 Fax. 972-2-561-9112 Email: [email protected] www.jcpa.org World Jewish Congress 9A Diskin Street, 5th Floor Kiryat Wolfson, Jerusalem 96440 Phone : +972 2 633 3000 Fax: +972 2 659 8100 Email: [email protected] www.worldjewishcongress.com Academic Editor: Ambassador Alan Baker Production Director: Ahuva Volk Graphic Design: Studio Rami & Jaki • www.ramijaki.co.il Cover Photos: Results from the United Nations vote, with signatures, November 29, 1947 (Israel State Archive) UN General Assembly Proclaims Establishment of the State of Israel, November 29, 1947 (Israel National Photo Collection) ISBN: 978-965-218-100-8 TABLE OF CONTENTS Introduction and Overview Ambassador Alan Baker .......................................................................................................................................................................... 5 The National Rights of Jews Professor Ruth Gavison ........................................................................................................................................................................... 9 “An Overwhelmingly Jewish State” - From the Balfour Declaration to the Palestine Mandate