Functional Connectivity in Cerebellar Cortex in Vivo
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Neural Control of Movement: Motor Neuron Subtypes, Proprioception and Recurrent Inhibition
List of Papers This thesis is based on the following papers, which are referred to in the text by their Roman numerals. I Enjin A, Rabe N, Nakanishi ST, Vallstedt A, Gezelius H, Mem- ic F, Lind M, Hjalt T, Tourtellotte WG, Bruder C, Eichele G, Whelan PJ, Kullander K (2010) Identification of novel spinal cholinergic genetic subtypes disclose Chodl and Pitx2 as mark- ers for fast motor neurons and partition cells. J Comp Neurol 518:2284-2304. II Wootz H, Enjin A, Wallen-Mackenzie Å, Lindholm D, Kul- lander K (2010) Reduced VGLUT2 expression increases motor neuron viability in Sod1G93A mice. Neurobiol Dis 37:58-66 III Enjin A, Leao KE, Mikulovic S, Le Merre P, Tourtellotte WG, Kullander K. 5-ht1d marks gamma motor neurons and regulates development of sensorimotor connections Manuscript IV Enjin A, Leao KE, Eriksson A, Larhammar M, Gezelius H, Lamotte d’Incamps B, Nagaraja C, Kullander K. Development of spinal motor circuits in the absence of VIAAT-mediated Renshaw cell signaling Manuscript Reprints were made with permission from the respective publishers. Cover illustration Carousel by Sasha Svensson Contents Introduction.....................................................................................................9 Background...................................................................................................11 Neural control of movement.....................................................................11 The motor neuron.....................................................................................12 Organization -

Molecular Plasticity of Adult Bergmann Fibers Is Associated with Radial Migration of Grafted Purkinje Cells
The Journal of Neuroscience, January 1994, 14(i): 124-133 Molecular Plasticity of Adult Bergmann Fibers Is Associated with Radial Migration of Grafted Purkinje Cells Constantino Sotelo,’ Rosa-Magda Alvarado-Mallart,i Monique Frain,2 and Muriel Verner ‘Neuromorphologie: Dkveloppement, Evolution, INSERM U. 106, HBpital de la Salpktrikre, 75651 Paris Cedex 13, France, *Biologic Molkulaire du Dkveloppement, INSERM U. 368 Ecole Normale Supkrieure, 75230 Paris Cedex, France, and 31nstitut Cochin de Gbnbtique Molkulaire, CJF 9003 INSERM, 75014 Paris, France Embryonic Purkinje cells (PCs) from cerebellar primordia adult cerebellum and become functionally integrated into the grafted in adult pcd mutant cerebellum replace missing PCs synaptic circuitry of the cerebellarcortex of the host (Sotelo and of the host, and become synaptically integrated into the de- Alvarado-Mallart, 1991). The cerebellar mutant mouse strains fective cerebellar circuit. This process of neuronal replace- .LJC~,nr, and Lc, in which Purkinje cells (PCs) die (Sidman and ment starts with the invasion of grafted PCs into the host Green, 1970; Mullen et al., 1976; Caddy and Biscoe, 1979; cerebellum, and their radial migration through its molecular Wassef et al., 1987; Dumesnil-Bousez and Sotelo, 1992), have layer. The present study is aimed at determining whether provided models for studying graft integration (Sotelo and Al- the glial axes for this migration are embryonic radial glial varado-Mallart, 1986, 1987a, 1991, 1992; Dumesnil-Bousezand cells that comigrate with the grafted PCs, or adult Bergmann Sotelo, 1993). Penetration of grafted PCs into the cerebellum fibers of the host, transiently reexpressing the molecular of mutant hosts begins 3-4 d after grafting (DAG 3-4) by tan- cues needed for their guidance of the migration. -

Innovations Present in the Primate Interneuron Repertoire
Article Innovations present in the primate interneuron repertoire https://doi.org/10.1038/s41586-020-2781-z Fenna M. Krienen1,2 ✉, Melissa Goldman1,2, Qiangge Zhang2,3, Ricardo C. H. del Rosario2, Marta Florio1,2, Robert Machold4, Arpiar Saunders1,2, Kirsten Levandowski2,3, Heather Zaniewski2,3, Received: 19 July 2019 Benjamin Schuman4, Carolyn Wu3, Alyssa Lutservitz1,2, Christopher D. Mullally1,2, Nora Reed1,2, Accepted: 1 July 2020 Elizabeth Bien1,2, Laura Bortolin1,2, Marian Fernandez-Otero2,5, Jessica D. Lin2, Alec Wysoker2, James Nemesh2, David Kulp2, Monika Burns5, Victor Tkachev6,7,8, Richard Smith9,10, Published online: xx xx xxxx Christopher A. Walsh9,10, Jordane Dimidschstein2, Bernardo Rudy4,11, Leslie S. Kean6,7,8, Check for updates Sabina Berretta5,12,13, Gord Fishell2,14, Guoping Feng2,3 & Steven A. McCarroll1,2 ✉ Primates and rodents, which descended from a common ancestor around 90 million years ago1, exhibit profound diferences in behaviour and cognitive capacity; the cellular basis for these diferences is unknown. Here we use single-nucleus RNA sequencing to profle RNA expression in 188,776 individual interneurons across homologous brain regions from three primates (human, macaque and marmoset), a rodent (mouse) and a weasel (ferret). Homologous interneuron types—which were readily identifed by their RNA-expression patterns—varied in abundance and RNA expression among ferrets, mice and primates, but varied less among primates. Only a modest fraction of the genes identifed as ‘markers’ of specifc interneuron subtypes in any one species had this property in another species. In the primate neocortex, dozens of genes showed spatial expression gradients among interneurons of the same type, which suggests that regional variation in cortical contexts shapes the RNA expression patterns of adult neocortical interneurons. -

Spinal Cord Organization
Lecture 4 Spinal Cord Organization The spinal cord . Afferent tract • connects with spinal nerves, through afferent BRAIN neuron & efferent axons in spinal roots; reflex receptor interneuron • communicates with the brain, by means of cell ascending and descending pathways that body form tracts in spinal white matter; and white matter muscle • gives rise to spinal reflexes, pre-determined gray matter Efferent neuron by interneuronal circuits. Spinal Cord Section Gross anatomy of the spinal cord: The spinal cord is a cylinder of CNS. The spinal cord exhibits subtle cervical and lumbar (lumbosacral) enlargements produced by extra neurons in segments that innervate limbs. The region of spinal cord caudal to the lumbar enlargement is conus medullaris. Caudal to this, a terminal filament of (nonfunctional) glial tissue extends into the tail. terminal filament lumbar enlargement conus medullaris cervical enlargement A spinal cord segment = a portion of spinal cord that spinal ganglion gives rise to a pair (right & left) of spinal nerves. Each spinal dorsal nerve is attached to the spinal cord by means of dorsal and spinal ventral roots composed of rootlets. Spinal segments, spinal root (rootlets) nerve roots, and spinal nerves are all identified numerically by th region, e.g., 6 cervical (C6) spinal segment. ventral Sacral and caudal spinal roots (surrounding the conus root medullaris and terminal filament and streaming caudally to (rootlets) reach corresponding intervertebral foramina) collectively constitute the cauda equina. Both the spinal cord (CNS) and spinal roots (PNS) are enveloped by meninges within the vertebral canal. Spinal nerves (which are formed in intervertebral foramina) are covered by connective tissue (epineurium, perineurium, & endoneurium) rather than meninges. -

Cerebellar Granule Cells in Culture
Proc. Nati. Acad. Sci. USA Vol. 83, pp. 4957-4961, July 1986 Neurobiology Cerebellar granule cells in culture: Monosynaptic connections with Purkinje cells and ionic currents (excitatory postsynaptic potential/patch-clamp) ToMoo HIRANO, YOSHIHIRo KUBO, AND MICHAEL M. WU Department of Neurobiology, Institute of Brain Research, School of Medicine, University of Tokyo, Tokyo, Japan Communicated by S. Hagiwara, March 6, 1986 ABSTRACT Electrophysiological properties of cerebellar tissue was dissociated by triturating with a fire-polished granule cells and synapses between granule and Purkinje cells Pasteur pipette in Ca-free Hanks' balanced salt solution were studied in dissociated cultures. Electrophysiological prop- containing 0.05% DNase and 12 mM MgSO4. The cells were erties of neurons and synapses in the mammalian central centrifuged at 150 x g at 40C and the pelleted cells were nervous system are best studied in dissociated cell cultures resuspended at a concentration of about 106 cells per ml in a because of good target cell visibility, control over the contents defined medium (9). One milliliter ofthe cell suspension from of the extracellular solution, and the feasibility of whole-cell newborn rats was plated first in a Petri dish (3.5 cm in patch electrode recording, which has been a powerful tech- diameter) containing several pieces ofheat-sterilized, poly(L- nique in analyzing biophysical properties of ionic channels in lysine)-coated coverslips, and then 1 ml of fetal cell suspen- small cells. We have applied this whole-cell recording technique sion was added. One-half of the culture medium was ex- to cultured cerebellar granule cells whose electrophysiological changed with fresh medium once a week. -
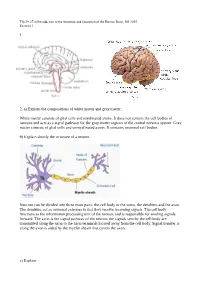
1. 2. A) Explain the Compositions of White Matter and Gray
Tfy-99.2710 Introduction to the Structure and Operation of the Human Brain, fall 2015 Exercise 1 1. 2. a) Explain the compositions of white matter and gray matter. White matter consists of glial cells and myelinated axons. It does not contain the cell bodies of neurons and acts as a signal pathway for the gray matter regions of the central nervous system. Gray matter consists of glial cells and unmyelinated axons. It contains neuronal cell bodies. b) Explain shortly the structure of a neuron. Neurons can be divided into three main parts: the cell body or the soma, the dendrites and the axon. The dendrites act as neuronal antennas in that they receive incoming signals. The cell body functions as the information processing unit of the neuron, and is responsible for sending signals forward. The axon is the signal pathway of the neuron; the signals sent by the cell body are transmitted along the axon to the axon terminals located away from the cell body. Signal transfer is along the axon is aided by the myelin sheath that covers the axon. c) Explain: Tfy-99.2710 Introduction to the Structure and Operation of the Human Brain, fall 2015 Exercise 1 i) myelin Membrane that wraps around axons. Formed from glial support cells: oligodendrogolia in the central nervous system and Schwann cells in the peripheral nervous system. Myelin facilitates signal transfer along axons by allowing action potentials to skip between the nodes of Ranvier (saltatory conduction). ii) receptor Molecule specialized in receiving a chemical signal by binding a specific neurotransmitter. -

Cortex Brainstem Spinal Cord Thalamus Cerebellum Basal Ganglia
Harvard-MIT Division of Health Sciences and Technology HST.131: Introduction to Neuroscience Course Director: Dr. David Corey Motor Systems I 1 Emad Eskandar, MD Motor Systems I - Muscles & Spinal Cord Introduction Normal motor function requires the coordination of multiple inter-elated areas of the CNS. Understanding the contributions of these areas to generating movements and the disturbances that arise from their pathology are important challenges for the clinician and the scientist. Despite the importance of diseases that cause disorders of movement, the precise function of many of these areas is not completely clear. The main constituents of the motor system are the cortex, basal ganglia, cerebellum, brainstem, and spinal cord. Cortex Basal Ganglia Cerebellum Thalamus Brainstem Spinal Cord In very broad terms, cortical motor areas initiate voluntary movements. The cortex projects to the spinal cord directly, through the corticospinal tract - also known as the pyramidal tract, or indirectly through relay areas in the brain stem. The cortical output is modified by two parallel but separate re entrant side loops. One loop involves the basal ganglia while the other loop involves the cerebellum. The final outputs for the entire system are the alpha motor neurons of the spinal cord, also called the Lower Motor Neurons. Cortex: Planning and initiation of voluntary movements and integration of inputs from other brain areas. Basal Ganglia: Enforcement of desired movements and suppression of undesired movements. Cerebellum: Timing and precision of fine movements, adjusting ongoing movements, motor learning of skilled tasks Brain Stem: Control of balance and posture, coordination of head, neck and eye movements, motor outflow of cranial nerves Spinal Cord: Spontaneous reflexes, rhythmic movements, motor outflow to body. -

Profound Functional and Molecular Diversity of Mitochondria Revealed by Cell Type-Specific Profiling in Vivo
bioRxiv preprint doi: https://doi.org/10.1101/403774; this version posted August 29, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Profound functional and molecular diversity of mitochondria revealed by cell type-specific profiling in vivo Authors: Caroline Fecher1,2,3,#, Laura Trovò1,2,#, Stephan A. Müller2,4, Nicolas Snaidero1,2, Jennifer Wettmarshausen5,6, Sylvia Heink7, Oskar Ortiz8,$, Ingrid Wagner9, Ralf Kühn8,$$, Jana Hartmann10, Rosa Maria Karl10, Arthur Konnerth10,11,12, Thomas Korn7,11, Wolfgang Wurst2,8,11,13, Doron Merkler9, Stefan F. Lichtenthaler2,4,11, Fabiana Perocchi5,6,11 and Thomas Misgeld1,2,11,12,* Affiliations: 1 Institute of Neuronal Cell Biology, Technical University of Munich, Germany 2 German Center for Neurodegenerative Diseases, Munich, Germany 3 Graduate School of Systemic Neuroscience, Ludwig-Maximilians University, Munich, Germany 4 Neuroproteomics, School of Medicine, Klinikum rechts der Isar, and Institute for Advanced Study, Technical University of Munich, Germany 5 Gene Center Munich, Ludwig-Maximilians University, Munich, Germany 6 Institute for Diabetes and Obesity, Helmholtz Zentrum München, Munich, Germany 7 Experimental Neuroimmunology, Klinikum rechts der Isar, Technical University of Munich, Germany 8 Institute of Developmental Genetics, Helmholtz Zentrum München, Munich, Germany 9 Department of -

Differences in the Developmental Patterns of Three Microtubule-Associated Proteins in the Rat Cerebellum’
0270.8474/85/0504-0977$02.00/O The Journal of Neuroscience Copyright 0 Society for Neuroscience Vol. 5, No. 4, pp. 977-991 Printed in U.S.A. April 1985 Differences in the Developmental Patterns of Three Microtubule-associated Proteins in the Rat Cerebellum’ ROBERT BERNHARDT,2 GERDA HUBER,3 AND ANDREW MATUS Friedrich Miescher-lnstitut, P. 0. Box 2543, CH-4002 Base/, Switzerland Abstract MAPS are potentially important physiological regulators of neuronal differentiation. The developmental distribution patternS of microtubule- It has become clear that microtubules of different tissues and cell associated proteins (MAPS) 1, 2, and 3 were studied using types are characterized by different MAPS. Thus, a category of high three monoclonal antibodies. lmmunochemical staining at the molecular weight MAPS, later identified as MAP2, is exclusively light and electron microscopic levels demonstrated the spe- expressed in adult neurons where it is most concentrated in den- cific localization of each MAP in different cellular and sub- drites (Matus et al., 1981, 1983; Caceres et al., 1983, 1984; Wiche cellular compartments. (i) MAPS, which is specifically asso- et al., 1983; Bernhardt and Matus, 1984; De Camilli et al., 1984). ciated with dendritic microtubules in the adult brain, is strictly This suggested the possibility that MAP2 could be specifically associated with growing dendrites from the onset of their involved in the formation of dendritic microtubules and hence could formation. (ii) MAP3, a recently described MAP of M, = play a significant role in the regulation of dendrite growth. This idea 180,000, which in the adult brain is associated with neurofi- found further support from immunohistochemical data of developing lament-rich axons and glial processes, is associated with brain (Bernhardt and Matus, 1982; Matus et al., 1983; Burgoyne and axons from the beginning of outgrowth. -

Lnteractions Between Cerebellar Purkinje Cells and Their Associated
Neuron-glia interactions of Purkinje cells is preserved in organotypic cerebellar astrocytes that is reflected in some neuron-glia cultures. As defined by Palay and Chan-Palay (1974), interactions that are not necessarily typical, but may be the sheaths are formed predominantly by processes of characteristic of more tightly coupled cells. With this Golgi epithelial cells, the astrocytes that give rise to the caveat in mind, we will examine the evidence for these radial Bergmann fibers that extend through the particular astrocytic properties. molecular layer of the cerebellar cortex and provide a lattice for granule cell migration (Rakic, 1971). These The cerebellar culture models astrocytes are often, though inappropriately, referred to as "Bergmann glia." Purkinje cells are the only neurons Parasagittally oriented cerebellar explants derived in the cerebellar cortex that are fully ensheathed by from newborn (within 24 hours after birth) Swiss- astrocytes, whereas basket and stellate cells have only Webster mice and maintained in Maximow chambers for partial sheaths and Golgi and granule cells (Fig. 3) are 2 or more weeks exhibit many of the structural and generally unensheathed (Palay and Chan-Palay, 1974). It functional characteristics of the cerebellum in vivo (Seil, is this close relationship between Purkinje cells and 1972; Leiman and Seil, 1973; reviewed in Seil, 1979, Fig. 1. Purkinje cell from an untreated control cerebellar culture, 15 DIV. Shown is the soma and a pottion of a dendrite (d) extending upward. The soma is surrounded by an astrocytic sheath, which appears as a relatively clear area immediately adjacent to the smoothly contoured soma membrane. Five axon terminals intenupt the sheath to contact the soma, two of which are synaptic contacts (large arrows). -

Intersegmental Interneurons Can Control the Gain of Reflexes in Adjacent Segments of the Locust by Their Action on Nonspiking Local Interneurons
The Journal of Neuroscience, September 1989, g(9): 30303039 Intersegmental Interneurons Can Control the Gain of Reflexes in Adjacent Segments of the Locust by Their Action on Nonspiking Local Interneurons Gilles Laurent and Malcolm Burrows Department of Zoology, University of Cambridge, Cambridge CB2 3EJ, England The gain of local reflexes of one leg of a locust can be altered partmentalized, with synaptic inputs and their associated by mechanosensory inputs generated by movements of or conductance changes restricted to particular branches. In tactile inputs to an adjacent leg. Touching the mesothoracic this way, an individual nonspiking neuron could contribute tarsus, for example, increases the number of spikes that are simultaneously to several local circuits. The inputs from dif- produced by the metathoracic slow extensor tibiae motor ferent intersegmental interneurons could then modulate these neuron and enhances the depolarization of flexor tibiae mo- pathways independently. tor neuron in response to imposed movements of the chor- dotonal organ in the ipsilateral hind femur. The sensory in- Nonspiking interneurons in the metathoracic ganglion of the formation from the middle leg is conveyed directly to locust receive direct inputs from mechanosensory afferents on nonspiking interneurons and motor neurons controlling the one hindleg and are essential elements in local reflex movements movements of the hindleg by a population of mesothoracic of that leg (Laurent and Burrows, 1988; Burrows et al., 1988). intersegmental interneurons (Laurent and Burrows, 1989). They also receive direct inputs from intersegmental intemeu- The metathoracic nonspiking interneurons receive direct in- rons that process the mechanosensory inputs from an ipsilateral puts from receptors on a hindleg and are, therefore, a point middle leg (Laurent and Burrows, 1989). -

Cerebellar Histology & Circuitry
Cerebellar Histology & Circuitry Histology > Neurological System > Neurological System CEREBELLAR HISTOLOGY & CIRCUITRY SUMMARY OVERVIEW Gross Anatomy • The folding of the cerebellum into lobes, lobules, and folia allows it to assume a tightly packed, inconspicuous appearance in the posterior fossa. • The cerebellum has a vast surface area, however, and when stretched, it has a rostrocaudal expanse of roughly 120 centimeters, which allows it to hold an estimated one hundred billion granule cells — more cells than exist within the entire cerebral cortex. - It is presumed that the cerebellum's extraordinary cell count plays an important role in the remarkable rehabilitation commonly observed in cerebellar stroke. Histology Two main classes of cerebellar nuclei • Cerebellar cortical neurons • Deep cerebellar nuclei CEREBELLAR CORTICAL CELL LAYERS Internal to external: Subcortical white matter Granule layer (highly cellular) • Contains granule cells, Golgi cells, and unipolar brush cells. Purkinje layer 1 / 9 • Single layer of large Purkinje cell bodies. • Purkinje cells project a fine axon through the granule cell layer. - Purkinje cells possess a large dendritic system that arborizes (branches) extensively and a single fine axon. Molecular layer • Primarily comprises cell processes but also contains stellate and basket cells. DEEP CEREBELLAR NUCLEI From medial to lateral: Fastigial Globose Emboliform Dentate The globose and emboliform nuclei are also known as the interposed nuclei • A classic acronym for the lateral to medial organization of the deep nuclei is "Don't Eat Greasy Food," for dentate, emboliform, globose, and fastigial. NEURONS/FUNCTIONAL MODULES • Fastigial nucleus plays a role in the vestibulo- and spinocerebellum. • Interposed nuclei are part of the spinocerebellum. • Dentate nucleus is part of the pontocerebellum.