MIGRATORY and RESIDENT WILD BIRDS and THEIR ROLE in the TRANSMISSION of Rickettsia Spp
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Habitat Associations of Ixodes Scapularis (Acari: Ixodidae) in Syracuse, New York
SUNY College of Environmental Science and Forestry Digital Commons @ ESF Honors Theses 5-2016 Habitat Associations of Ixodes Scapularis (Acari: Ixodidae) in Syracuse, New York Brigitte Wierzbicki Follow this and additional works at: https://digitalcommons.esf.edu/honors Part of the Entomology Commons Recommended Citation Wierzbicki, Brigitte, "Habitat Associations of Ixodes Scapularis (Acari: Ixodidae) in Syracuse, New York" (2016). Honors Theses. 106. https://digitalcommons.esf.edu/honors/106 This Thesis is brought to you for free and open access by Digital Commons @ ESF. It has been accepted for inclusion in Honors Theses by an authorized administrator of Digital Commons @ ESF. For more information, please contact [email protected], [email protected]. HABITAT ASSOCIATIONS OF IXODES SCAPULARIS (ACARI: IXODIDAE) IN SYRACUSE, NEW YORK By Brigitte Wierzbicki Candidate for Bachelor of Science Environmental and Forest Biology With Honors May,2016 APPROVED Thesis Project Advisor: Af ak Ck M issa K. Fierke, Ph.D. Second Reader: ~~ Nicholas Piedmonte, M.S. Honors Director: w44~~d. William M. Shields, Ph.D. Date: ~ / b / I & r I II © 2016 Copyright B. R. K. Wierzbicki All rights reserved. 111 ABSTRACT Habitat associations of Jxodes scapularis Say were described at six public use sites within Syracuse, New York. Adult, host-seeking blacklegged ticks were collected using tick flags in October and November, 2015 along two 264 m transects at each site, each within a distinct forest patch. We examined the association of basal area, leaf litter depth, and percent understory cover with tick abundance using negative binomial regression models. Models indicated tick abundance was negatively associated with percent understory cover, but was not associated with particular canopy or understory species. -

Ticks (Parasitiformes: Ixodida) on New World Wild Primates in Brazil
International Journal of Acarology ISSN: (Print) (Online) Journal homepage: https://www.tandfonline.com/loi/taca20 Ticks (Parasitiformes: Ixodida) on new world wild primates in Brazil Thiago F. Martins, Rodrigo H. F. Teixeira, Julio C. Souza Jr, Hermes R. Luz, Mônica M. Montenegro, Leandro Jerusalinsky, Marina G. Bueno, Valeria C. Onofrio, Marinete Amorim, Gilberto S. Gazêta, Paula De J. Da Silva, Karla Bitencourth, Ana B. P. Borsoi, Sandro Marques, Marco O. Mattos Jr, Leandra S. I. Hernandes, Alessandra Scofild, Rafael F. C. Vieira, Richard C. Pacheco, Maurício C. Horta, Valéria P. da Silva, Patrícia W. Silva, Claudia A. Igayara, Thais C. Sanches, Marcello S. Nardi, Camile Lugarini, Natasha L. Maia, Cláudio L. M. de Siqueira, Juliana M. Ferreira, João F. Soares & Marcelo B. Labruna To cite this article: Thiago F. Martins, Rodrigo H. F. Teixeira, Julio C. Souza Jr, Hermes R. Luz, Mônica M. Montenegro, Leandro Jerusalinsky, Marina G. Bueno, Valeria C. Onofrio, Marinete Amorim, Gilberto S. Gazêta, Paula De J. Da Silva, Karla Bitencourth, Ana B. P. Borsoi, Sandro Marques, Marco O. Mattos Jr, Leandra S. I. Hernandes, Alessandra Scofild, Rafael F. C. Vieira, Richard C. Pacheco, Maurício C. Horta, Valéria P. da Silva, Patrícia W. Silva, Claudia A. Igayara, Thais C. Sanches, Marcello S. Nardi, Camile Lugarini, Natasha L. Maia, Cláudio L. M. de Siqueira, Juliana M. Ferreira, João F. Soares & Marcelo B. Labruna (2021): Ticks (Parasitiformes: Ixodida) on new world wild primates in Brazil, International Journal of Acarology, DOI: 10.1080/01647954.2020.1870554 To link to this article: https://doi.org/10.1080/01647954.2020.1870554 Published online: 03 Mar 2021. -

Redalyc.Ticks Infesting Wild Small Rodents in Three Areas of the State Of
Ciência Rural ISSN: 0103-8478 [email protected] Universidade Federal de Santa Maria Brasil Fernandes Martins, Thiago; Gea Peres, Marina; Borges Costa, Francisco; Silva Bacchiega, Thais; Appolinario, Camila Michele; Azevedo de Paula Antunes, João Marcelo; Ferreira Vicente, Acácia; Megid, Jane; Bahia Labruna, Marcelo Ticks infesting wild small rodents in three areas of the state of São Paulo, Brazil Ciência Rural, vol. 46, núm. 5, mayo, 2016, pp. 871-875 Universidade Federal de Santa Maria Santa Maria, Brasil Available in: http://www.redalyc.org/articulo.oa?id=33144653018 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Ciência Rural, Santa Maria, v.46,Ticks n.5, infesting p.871-875, wild mai, small 2016 rodents in three areas of the state of http://dx.doi.org/10.1590/0103-8478cr20150671São Paulo, Brazil. 871 ISSN 1678-4596 PARASITOLOGY Ticks infesting wild small rodents in three areas of the state of São Paulo, Brazil Carrapatos infestando pequenos roedores silvestres em três municípios do estado de São Paulo, Brasil Thiago Fernandes MartinsI* Marina Gea PeresII Francisco Borges CostaI Thais Silva BacchiegaII Camila Michele AppolinarioII João Marcelo Azevedo de Paula AntunesII Acácia Ferreira VicenteII Jane MegidII Marcelo Bahia LabrunaI ABSTRACT carrapatos, os quais foram coletados e identificados ao nível de espécie em laboratório, através de análises morfológicas (para From May to September 2011, a total of 138 wild adultos, ninfas e larvas) e por biologia molecular para confirmar rodents of the Cricetidae family were collected in the cities of estas análises, através do sequenciamento de um fragmento Anhembi, Bofete and Torre de Pedra, in São Paulo State. -
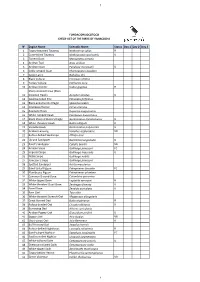
N° English Name Scientific Name Status Day 1
1 FUNDACIÓN JOCOTOCO CHECK-LIST OF THE BIRDS OF YANACOCHA N° English Name Scientific Name Status Day 1 Day 2 Day 3 1 Tawny-breasted Tinamou Nothocercus julius R 2 Curve-billed Tinamou Nothoprocta curvirostris U 3 Torrent Duck Merganetta armata 4 Andean Teal Anas andium 5 Andean Guan Penelope montagnii U 6 Sickle-winged Guan Chamaepetes goudotii 7 Cattle Egret Bubulcus ibis 8 Black Vulture Coragyps atratus 9 Turkey Vulture Cathartes aura 10 Andean Condor Vultur gryphus R Sharp-shinned Hawk (Plain- 11 breasted Hawk) Accipiter striatus U 12 Swallow-tailed Kite Elanoides forficatus 13 Black-and-chestnut Eagle Spizaetus isidori 14 Cinereous Harrier Circus cinereus 15 Roadside Hawk Rupornis magnirostris 16 White-rumped Hawk Parabuteo leucorrhous 17 Black-chested Buzzard-Eagle Geranoaetus melanoleucus U 18 White-throated Hawk Buteo albigula R 19 Variable Hawk Geranoaetus polyosoma U 20 Andean Lapwing Vanellus resplendens VR 21 Rufous-bellied Seedsnipe Attagis gayi 22 Upland Sandpiper Bartramia longicauda R 23 Baird's Sandpiper Calidris bairdii VR 24 Andean Snipe Gallinago jamesoni FC 25 Imperial Snipe Gallinago imperialis U 26 Noble Snipe Gallinago nobilis 27 Jameson's Snipe Gallinago jamesoni 28 Spotted Sandpiper Actitis macularius 29 Band-tailed Pigeon Patagoienas fasciata FC 30 Plumbeous Pigeon Patagioenas plumbea 31 Common Ground-Dove Columbina passerina 32 White-tipped Dove Leptotila verreauxi R 33 White-throated Quail-Dove Zentrygon frenata U 34 Eared Dove Zenaida auriculata U 35 Barn Owl Tyto alba 36 White-throated Screech-Owl Megascops -

Genus Boophilus Curtice Genus Rhipicentor Nuttall & Warburton
3 CONTENTS General remarks 4 Genus Amblyomma Koch 5 Genus Anomalohimalaya Hoogstraal, Kaiser & Mitchell 46 Genus Aponomma Neumann 47 Genus Boophilus Curtice 58 Genus Hyalomma Koch. 63 Genus Margaropus Karsch 82 Genus Palpoboophilus Minning 84 Genus Rhipicentor Nuttall & Warburton 84 Genus Uroboophilus Minning. 84 References 86 SUMMARI A list of species and subspecies currently included in the tick genera Amblyomma, Aponomma, Anomalohimalaya, Boophilus, Hyalomma, Margaropus, and Rhipicentor, as well as in the unaccepted genera Palpoboophilus and Uroboophilus is given in this paper. The published synonymies and authors of each spécifie or subspecific name are also included. Remaining tick genera have been reviewed in part in a previous paper of this series, and will be finished in a future third part. Key-words: Amblyomma, Aponomma, Anomalohimalaya, Boophilus, Hyalomma, Margaropus, Rhipicentor, Uroboophilus, Palpoboophilus, species, synonymies. RESUMEN Se proporciona una lista de las especies y subespecies actualmente incluidas en los géneros Amblyomma, Aponomma, Anomalohimalaya, Boophilus, Hyalomma, Margaropus y Rhipicentor, asi como en los géneros no aceptados Palpoboophilus and Uroboophilus. Se incluyen también las sinonimias publicadas y los autores de cada nombre especifico o subespecifico. Los restantes géneros de garrapatas han sido revisados en parte en un volumen previo de esta serie, y serân terminados en una futura tercera parte. Palabras claves Amblyomma, Aponomma, Anomalohimalaya, Boophilus, Hyalomma, Margaropus, Rhipicentor, Uroboophilus, Palpoboophilus, especies, sinonimias. 4 GENERAL REMARKS Following is a list of species and subspecies of ticks d~e scribed in the genera Amblyomma, Aponomma, Anomalohimalaya, Boophilus, Hyalorma, Margaropus, and Rhipicentor, as well as in the unaccepted genera Palpoboophilus and Uroboophilus. The first volume (Estrada- Pena, 1991) included data for Haemaphysalis, Anocentor, Dermacentor, and Cosmiomma. -

Redalyc.Ticks on Birds from Cerrado Forest Patches Along The
Ciência Rural ISSN: 0103-8478 [email protected] Universidade Federal de Santa Maria Brasil Torga, Khelma; Tolesano-Pascoli, Graziela; Bonfim Vasquez, Jacqueline; da Silva Júnior, Eurípedes Luciano; Bahia Labruna, Marcelo; Fernandes Martins, Thiago; Ogrzewalska, Maria; Szabó, Matias Pablo Juan Ticks on birds from Cerrado forest patches along the Uberabinha river in the Triângulo Mineiro region of Minas Gerais, Brazil Ciência Rural, vol. 43, núm. 10, octubre, 2013, pp. 1852-1857 Universidade Federal de Santa Maria Santa Maria, Brasil Available in: http://www.redalyc.org/articulo.oa?id=33128114019 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Ciência1852 Rural, Santa Maria, v.43, n.10, p.1852-1857, out, 2013 Torga et al. ISSN 0103-8478 Ticks on birds from Cerrado forest patches along the Uberabinha river in the Triângulo Mineiro region of Minas Gerais, Brazil Carrapatos em aves em matas de cerrado ao longo do rio Uberabinha na região do Triângulo Mineiro de Minas Gerais, Brasil Khelma TorgaI Graziela Tolesano-PascoliI Jacqueline Bonfi m VasquezI Eurípedes Luciano da Silva JúniorI Marcelo Bahia LabrunaII Thiago Fernandes MartinsII Maria OgrzewalskaII Matias Pablo Juan SzabóI* ABSTRACT (50%). A intensidade média de infestação por carrapatos foi baixa (1,8 carrapato por ave infestada) e a maior parte dos parasitas We herein describe ticks parasitizing birds in forest recolhidos estava localizada no pescoço (60%) das aves, seguido fragments along the Uberabinha River, a major watercourse pela cabeça (20%). -

Dacninae Species Tree, Part I
Dacninae I: Nemosiini, Conirostrini, & Diglossini Hooded Tanager, Nemosia pileata Cherry-throated Tanager, Nemosia rourei Nemosiini Blue-backed Tanager, Cyanicterus cyanicterus White-capped Tanager, Sericossypha albocristata Scarlet-throated Tanager, Sericossypha loricata Bicolored Conebill, Conirostrum bicolor Pearly-breasted Conebill, Conirostrum margaritae Chestnut-vented Conebill, Conirostrum speciosum Conirostrini White-eared Conebill, Conirostrum leucogenys Capped Conebill, Conirostrum albifrons Giant Conebill, Conirostrum binghami Blue-backed Conebill, Conirostrum sitticolor White-browed Conebill, Conirostrum ferrugineiventre Tamarugo Conebill, Conirostrum tamarugense Rufous-browed Conebill, Conirostrum rufum Cinereous Conebill, Conirostrum cinereum Stripe-tailed Yellow-Finch, Pseudochloris citrina Gray-hooded Sierra Finch, Phrygilus gayi Patagonian Sierra Finch, Phrygilus patagonicus Peruvian Sierra Finch, Phrygilus punensis Black-hooded Sierra Finch, Phrygilus atriceps Gough Finch, Rowettia goughensis White-bridled Finch, Melanodera melanodera Yellow-bridled Finch, Melanodera xanthogramma Inaccessible Island Finch, Nesospiza acunhae Nightingale Island Finch, Nesospiza questi Wilkins’s Finch, Nesospiza wilkinsi Saffron Finch, Sicalis flaveola Grassland Yellow-Finch, Sicalis luteola Orange-fronted Yellow-Finch, Sicalis columbiana Sulphur-throated Finch, Sicalis taczanowskii Bright-rumped Yellow-Finch, Sicalis uropigyalis Citron-headed Yellow-Finch, Sicalis luteocephala Patagonian Yellow-Finch, Sicalis lebruni Greenish Yellow-Finch, -

Amblyomma Nodosum) in an Endemic Area of Spotted Fever in Brazil Author(S): Leonardo Moerbeck, Vinicius F
Rickettsia sp. Strain NOD Infecting Ticks (Amblyomma nodosum) in an Endemic Area of Spotted Fever in Brazil Author(s): Leonardo Moerbeck, Vinicius F. Vizzoni, Stefan V. de Oliveira, Robson Cavalcante, Gerlene C. B. Coelho, Naylê F. H. Duarte, Marinete Amorim, and Gilberto S. Gazêta Source: Journal of Wildlife Diseases, 54(2):406-409. Published By: Wildlife Disease Association https://doi.org/10.7589/2017-06-137 URL: http://www.bioone.org/doi/full/10.7589/2017-06-137 BioOne (www.bioone.org) is a nonprofit, online aggregation of core research in the biological, ecological, and environmental sciences. BioOne provides a sustainable online platform for over 170 journals and books published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Web site, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/page/ terms_of_use. Usage of BioOne content is strictly limited to personal, educational, and non-commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. DOI: 10.7589/2017-06-137 Journal of Wildlife Diseases, 54(2), 2018, pp. 406–409 Ó Wildlife Disease Association 2018 Rickettsia sp. Strain NOD Infecting Ticks (Amblyomma nodosum)inan Endemic Area of Spotted Fever in Brazil Leonardo Moerbeck,1,2 Vinicius F. Vizzoni,2 Stefan V. de Oliveira,3 Robson Cavalcante,4 Gerlene C. -

Notes on the Plumages of the Paramo Seedeater (Catamenia Homochroa)
January1986] ShortCommunications 227 shortcomingof the modal method is that it estimates -, & J. T. EMLEN. 1966. A technique for record- only the direction of activity and gives no indication ing migratory orientation of captive birds. Auk of the scatterof hopping, whereas the traditional 83: 361-367. method indicates the degree of concentration of a HAMILTONßW. J., III. 1966. Analysis of bird navi- bird's hopping (mean vector length, r). This is not gationexperiments. Pp. 147-178in Systemsanal- often a major problem becausemost recent studies ysis in ecology (K. E. F. Watt, Ed.). New York, have relied on second-orderanalyses of mean direc- Academic Press. tions that ignore the mean vector lengths. Indeed, at MARDIA, K. V. 1972. Statistics of directional data. this point the biological meaning of the dispersion New York, Academic Press. of a bird's hoppingactivity is not clearand is in part MOORE,F. R. 1985. Individual variability in the mi- a reflection of the idiosyncraciesof individual birds gratory orientation of the Savannah Sparrow, (Wiltschko and Wiltschko 1978, Moore 1985). Passerculussandwichensis. Z. Tierpsychol. 67: 144- 153. LITERATURE CITED RABOL,J. 1969. Orientation of autumn migrating Garden Warblers (Sylvia borin) after displace- BATSCHELET,E. 1978. Second-order statistical anal- ment from western Denmark (Blfivand) to east- ysisof directions.Pp. 3-24 in Animal migration, ern Sweden (Ottenby). A preliminary experi- navigation, and homing (K. Schmidt-Koenig and ment. Dansk Ornithol. Foren. Tids. 63: 93-104. W. T. Keeton, Eds.).Berlin, Springer-Verlag. 1970. Transformationof colour degreesto 1981. Circular statisticsin biology. New number of jumps using the Emlen orientation York, Academic Press. -

Brown Dog Tick, Rhipicephalus Sanguineus Latreille (Arachnida: Acari: Ixodidae)1 Yuexun Tian, Cynthia C
EENY-221 Brown Dog Tick, Rhipicephalus sanguineus Latreille (Arachnida: Acari: Ixodidae)1 Yuexun Tian, Cynthia C. Lord, and Phillip E. Kaufman2 Introduction and already-infested residences. The infestation can reach high levels, seemingly very quickly. However, the early The brown dog tick, Rhipicephalus sanguineus Latreille, has stages of the infestation, when only a few individuals are been found around the world. Many tick species can be present, are often missed completely. The first indication carried indoors on animals, but most cannot complete their the dog owner has that there is a problem is when they start entire life cycle indoors. The brown dog tick is unusual noticing ticks crawling up the walls or on curtains. among ticks, in that it can complete its entire life cycle both indoors and outdoors. Because of this, brown dog tick infestations can develop in dog kennels and residences, as well as establish populations in colder climates (Dantas- Torres 2008). Although brown dog ticks will feed on a wide variety of mammals, dogs are the preferred host in the United States and appear to be a necessary condition for maintaining a large tick populations (Dantas-Torres 2008). Brown dog tick management is important as they are a vector of several pathogens that cause canine and human diseases. Brown dog tick populations can be managed with habitat modification and pesticide applications. The taxonomy of the brown dog tick is currently under review Figure 1. Life stages of the brown dog tick, Rhipicephalus sanguineus and ultimately it may be determined that there are more Latreille. Clockwise from bottom right: engorged larva, engorged than one species causing residential infestations world-wide nymph, female, and male. -

Morphometrics of Amblyomma Mixtum in the State of Veracruz, Mexico
pathogens Article Morphometrics of Amblyomma mixtum in the State of Veracruz, Mexico Mariel Aguilar-Domínguez 1, Dora Romero-Salas 1,* , Sokani Sánchez-Montes 2, Ricardo Serna-Lagunes 3 , Greta Rosas-Saito 4, Anabel Cruz-Romero 1 and Adalberto A. Pérez de León 5,6 1 Laboratorio de Parasitología, rancho “Torreón del Molino”, Facultad de Medicina Veterinaria y Zootecnia, Universidad Veracruzana, Veracruz 91697, Mexico; [email protected] (M.A.-D.); [email protected] (A.C.-R.) 2 Facultad de Ciencias Biológicas y Agropecuarias Región Tuxpan, Universidad Veracruzana, Tuxpam 92870, Mexico; [email protected] 3 Laboratorio de Bioinformática y Bioestadística, Facultad de Ciencias Biológicas y Agropecuarias, Universidad Veracruzana, Córdoba 94945, Mexico; [email protected] 4 Red de Estudios Moleculares Avanzados, Instituto de Ecología, Xalapa 91073, Mexico; [email protected] 5 USDA-ARS Knipling-Bushland U.S. Veterinary Pest Genomics Center and Livestock Insects Research Laboratory, Kerrville, TX 78028, USA; [email protected] 6 USDA-ARS San Joaquin Valley Agricultural Sciences Center, Parlier, CA 93648, USA * Correspondence: [email protected]; Tel.: +52-(229)-9342075 Abstract: The tick Amblyomma mixtum is an ectoparasite of veterinary and public health importance because of its role as a vector of zoonotic pathogens. However, little is known about A. mixtum intraspecific variability and if morphological differentiation exists between populations across its geographic range. This study aimed to determine by electron microscopy the morphological vari- ability of A. mixtum populations in the state of Veracruz, which has a large livestock population Citation: Aguilar-Domínguez, M.; among states in Mexico. Forty male and 40 female A. mixtum collected from the 10 natural regions Romero-Salas, D.; Sánchez-Montes, S.; of Veracruz state were analyzed microscopically to accomplish main component analysis for each Serna-Lagunes, R.; Rosas-Saito, G.; sex. -

21 Sep 2018 Lists of Victims and Hosts of the Parasitic
version: 21 Sep 2018 Lists of victims and hosts of the parasitic cowbirds (Molothrus). Peter E. Lowther, Field Museum Brood parasitism is an awkward term to describe an interaction between two species in which, as in predator-prey relationships, one species gains at the expense of the other. Brood parasites "prey" upon parental care. Victimized species usually have reduced breeding success, partly because of the additional cost of caring for alien eggs and young, and partly because of the behavior of brood parasites (both adults and young) which may directly and adversely affect the survival of the victim's own eggs or young. About 1% of all bird species, among 7 families, are brood parasites. The 5 species of brood parasitic “cowbirds” are currently all treated as members of the genus Molothrus. Host selection is an active process. Not all species co-occurring with brood parasites are equally likely to be selected nor are they of equal quality as hosts. Rather, to varying degrees, brood parasites are specialized for certain categories of hosts. Brood parasites may rely on a single host species to rear their young or may distribute their eggs among many species, seemingly without regard to any characteristics of potential hosts. Lists of species are not the best means to describe interactions between a brood parasitic species and its hosts. Such lists do not necessarily reflect the taxonomy used by the brood parasites themselves nor do they accurately reflect the complex interactions within bird communities (see Ortega 1998: 183-184). Host lists do, however, offer some insight into the process of host selection and do emphasize the wide variety of features than can impact on host selection.