Monitoring the Northern Population of Copperbelly Water Snake (Nerodia Erythrogaster Neglecta) Using Occupancy Estimation and Modeling to Inform Conservation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Yellow-Bellied Water Snake Plain-Bellied Water
Nature Flashcards Snakes All photos are subject to the terms of the Creative Commons Public License Based on Nature Quiz Attribution-Non-Commercial 3.0 United States unless copyright otherwise By Phil Huxford noted. TMN-COT Meeting November, 2013 Texas Master Naturalist Cradle of Texas Chapter Cradle of Texas Chapter Yellow-bellied Water Snake Plain-bellied Water Snake Nerodia erythrogaster flavigaster Elliptical eye pupils Bright yellow underneath Found around ponds, lakes, swamps, and wet bottomland forests 2 – 3 feet long Cradle of Texas Chapter Broad-banded Water snake Nerodia fasciata confluens Dark, wide bands separated by yellow Bold, dark checked stripes Strong swimmer Cradle of Texas Chapter 2 – 4 feet long Blotched Water Snake Nerodia erythrogaster transversa Black-edged; dark brown dorsal markings Yellow or sometimes orange belly Lives in small ponds, ditches, and rain-filled pools Typically 2 – 5 feet long Cradle of Texas Chapter Diamond-back Water Snake Northern Diamond-back Water Snake Nerodia rhombifer Heavy-bodied, large girth Can be dark brown Head somewhat flattened and wide Texas’ largest Nerodia Strikes without warning and viciously 4 – 6’ long Cradle of Texas Chapter Photo by J.D. Wilson http://srelherp.uga.edu/snakes/ Western Mud Snake Mud Snake Farancia abacura Lives in our area but rarely seen Glossy black above Red belly with black lines in belly Found in wooded swampland and wet areas Does not bite when handled but pokes tail like stinger 3 – 4 feet long Cradle of Texas Chapter Texas Coral Snake Micrurus fulvius tenere Blunt head; shiny, slender body Round pupils Colors red, yellow, black Lives in partly wooded organic material Cradle of Texas Chapter Usually 2 – 3 feet long Record: 47 ¾ inches in Brazoria County ‘Red touches yellow – kill a fellow. -

Check List 17 (1): 27–38
17 1 ANNOTATED LIST OF SPECIES Check List 17 (1): 27–38 https://doi.org/10.15560/17.1.27 A herpetological survey of Edith L. Moore Nature Sanctuary Dillon Jones1, Bethany Foshee2, Lee Fitzgerald1 1 Biodiversity Research and Teaching Collections, Department of Ecology and Conservation Biology, Texas A&M University, College Station, TX, USA. 2 Houston Audubon, 440 Wilchester Blvd. Houston, TX 77079 USA. Corresponding author: Dillon Jones, [email protected] Abstract Urban herpetology deals with the interaction of amphibians and reptiles with each other and their environment in an ur- ban setting. As such, well-preserved natural areas within urban environments can be important tools for conservation. Edith L. Moore Nature Sanctuary is an 18-acre wooded sanctuary located west of downtown Houston, Texas and is the headquarters to Houston Audubon Society. This study compared iNaturalist data with results from visual encounter surveys and aquatic funnel traps. Results from these two sources showed 24 species belonging to 12 families and 17 genera of herpetofauna inhabit the property. However, several species common in surrounding areas were absent. Combination of data from community science and traditional survey methods allowed us to better highlight herpe- tofauna present in the park besides also identifying species that may be of management concern for Edith L. Moore. Keywords Community science, iNaturalist, urban herpetology Academic editor: Luisa Diele-Viegas | Received 27 August 2020 | Accepted 16 November 2020 | Published 6 January 2021 Citation: Jones D, Foshee B, Fitzgerald L (2021) A herpetology survey of Edith L. Moore Nature Sanctuary. Check List 17 (1): 27–28. https://doi. -

Northern Population Segment of the Copperbelly Water Snake (Nerodia Erythrogaster Neglecta) Recovery Plan
U. S. Fish and Wildlife Service Northern Population Segment of the Copperbelly Water Snake (Nerodia erythrogaster neglecta) Recovery Plan December 2008 Department of the Interior U.S. Fish and Wildlife Service Midwest Region Fort Snelling, MN Cover graphic The drawing of Nerodia erythrogaster is reproduced with permission of the authors from the book, North American Watersnakes: A Natural History, by J. Whitfield Gibbons and Michael E. Dorcas, published by the University of Oklahoma Press (2004). Drawing by Peri Mason, Savannah River Ecology Laboratory. DISCLAIMER Recovery plans delineate reasonable actions which are believed to be required to recover and/or conserve listed species. Plans are published by the U.S. Fish and Wildlife Service (Service), sometimes prepared with the assistance of recovery teams, contractors, state agencies, and others. Objectives will be attained and any necessary funds made available subject to budgetary and other constraints affecting the parties involved, as well as the need to address other priorities. Recovery plans do not necessarily represent the views or the official positions or approval of any individuals or agencies involved in the plan formulation, other than the Service. They represent the official position of the Service only after they have been signed by the Regional Director. Approved recovery plans are subject to modification as dictated by new findings, changes in species status, and the completion of recovery actions. LITERATURE CITATION SHOULD READ AS FOLLOWS: U.S. Fish and Wildlife Service. 2008. Northern Population Segment of the Copperbelly Water Snake (Nerodia erythrogaster neglecta) Recovery Plan. Fort Snelling, Minnesota. ix + 79 pp. ADDITIONAL COPIES OF THIS PLAN CAN BE OBTAINED FROM: U.S. -

Response of Reptile and Amphibian Communities to the Reintroduction of Fire T in an Oak/Hickory Forest ⁎ Steven J
Forest Ecology and Management 428 (2018) 1–13 Contents lists available at ScienceDirect Forest Ecology and Management journal homepage: www.elsevier.com/locate/foreco Response of reptile and amphibian communities to the reintroduction of fire T in an oak/hickory forest ⁎ Steven J. Hromadaa, , Christopher A.F. Howeyb,c, Matthew B. Dickinsond, Roger W. Perrye, Willem M. Roosenburgc, C.M. Giengera a Department of Biology and Center of Excellence for Field Biology, Austin Peay State University, Clarksville, TN 37040, United States b Biology Department, University of Scranton, Scranton, PA 18510, United States c Ohio Center for Ecology and Evolutionary Studies, Department of Biological Sciences, Ohio University, Athens, OH 45701, United States d Northern Research Station, U.S. Forest Service, Delaware, OH 43015, United States e Southern Research Station, U.S. Forest Service, Hot Springs, AR 71902, United States ABSTRACT Fire can have diverse effects on ecosystems, including direct effects through injury and mortality and indirect effects through changes to available resources within the environment. Changes in vegetation structure suchasa decrease in canopy cover or an increase in herbaceous cover from prescribed fire can increase availability of preferred microhabitats for some species while simultaneously reducing preferred conditions for others. We examined the responses of herpetofaunal communities to prescribed fires in an oak/hickory forest in western Kentucky. Prescribed fires were applied twice to a 1000-ha area one and four years prior to sampling, causing changes in vegetation structure. Herpetofaunal communities were sampled using drift fences, and vegetation attributes were sampled via transects in four burned and four unburned plots. Differences in reptile community structure correlated with variation in vegetation structure largely created by fires. -
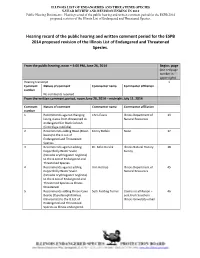
2014 ESPB Proposed List Revision Hearing and Written Comment
ILLINOIS LIST OF ENDANGERED AND THREATENED SPECIES 5-YEAR REVIEW AND REVISION ENDING IN 2014 Public Hearing Documents: Hearing record of the public hearing and written comment period for the ESPB 2014 proposed revision of the Illinois List of Endangered and Threatened Species. Hearing record of the public hearing and written comment period for the ESPB 2014 proposed revision of the Illinois List of Endangered and Threatened Species. From the public hearing, noon – 4:00 PM, June 26, 2014 Begins page (use red page number in upper right) Hearing transcript 1 Comment Nature of comment Commenter name Commenter affiliation number No comments received. From the written comment period, noon June 26, 2014 – midnight July 11, 2014 Comment Nature of comment Commenter name Commenter affiliation number 1 Recommends against changing Chris Evans Illinois Department of 13 listing status from threatened to Natural Resources endangered for Black Cohosh (Cimicifuga rubifolia). 2 Recommends adding Bison (Bison Kenny Bielski None 17 bison) to the IL List of Endangered and Threatened Species. 3 Recommends against adding Dr. Mike Dreslik Illinois Natural History 18 Copperbelly Water Snake Survey (Nerodia erythrogaster neglecta) to the IL List of Endangered and Threatened Species. 4 Recommends against adding Ann Holtrop Illinois Department of 45 Copperbelly Water Snake Natural Resources (Nerodia erythrogaster neglecta) to the IL List of Endangered and Threatened Species as Illinois threatened. 5 Recommends adding Illinois Cave Seth Fielding Turner Claims no -

Notice Warning Concerning Copyright Restrictions P.O
Publisher of Journal of Herpetology, Herpetological Review, Herpetological Circulars, Catalogue of American Amphibians and Reptiles, and three series of books, Facsimile Reprints in Herpetology, Contributions to Herpetology, and Herpetological Conservation Officers and Editors for 2015-2016 President AARON BAUER Department of Biology Villanova University Villanova, PA 19085, USA President-Elect RICK SHINE School of Biological Sciences University of Sydney Sydney, AUSTRALIA Secretary MARION PREEST Keck Science Department The Claremont Colleges Claremont, CA 91711, USA Treasurer ANN PATERSON Department of Natural Science Williams Baptist College Walnut Ridge, AR 72476, USA Publications Secretary BRECK BARTHOLOMEW Notice warning concerning copyright restrictions P.O. Box 58517 Salt Lake City, UT 84158, USA Immediate Past-President ROBERT ALDRIDGE Saint Louis University St Louis, MO 63013, USA Directors (Class and Category) ROBIN ANDREWS (2018 R) Virginia Polytechnic and State University, USA FRANK BURBRINK (2016 R) College of Staten Island, USA ALISON CREE (2016 Non-US) University of Otago, NEW ZEALAND TONY GAMBLE (2018 Mem. at-Large) University of Minnesota, USA LISA HAZARD (2016 R) Montclair State University, USA KIM LOVICH (2018 Cons) San Diego Zoo Global, USA EMILY TAYLOR (2018 R) California Polytechnic State University, USA GREGORY WATKINS-COLWELL (2016 R) Yale Peabody Mus. of Nat. Hist., USA Trustee GEORGE PISANI University of Kansas, USA Journal of Herpetology PAUL BARTELT, Co-Editor Waldorf College Forest City, IA 50436, USA TIFFANY -

Amphibians and Reptiles of the State of Coahuila, Mexico, with Comparison with Adjoining States
A peer-reviewed open-access journal ZooKeys 593: 117–137Amphibians (2016) and reptiles of the state of Coahuila, Mexico, with comparison... 117 doi: 10.3897/zookeys.593.8484 CHECKLIST http://zookeys.pensoft.net Launched to accelerate biodiversity research Amphibians and reptiles of the state of Coahuila, Mexico, with comparison with adjoining states Julio A. Lemos-Espinal1, Geoffrey R. Smith2 1 Laboratorio de Ecología-UBIPRO, FES Iztacala UNAM. Avenida los Barrios 1, Los Reyes Iztacala, Tlalnepantla, edo. de México, Mexico – 54090 2 Department of Biology, Denison University, Granville, OH, USA 43023 Corresponding author: Julio A. Lemos-Espinal ([email protected]) Academic editor: A. Herrel | Received 15 March 2016 | Accepted 25 April 2016 | Published 26 May 2016 http://zoobank.org/F70B9F37-0742-486F-9B87-F9E64F993E1E Citation: Lemos-Espinal JA, Smith GR (2016) Amphibians and reptiles of the state of Coahuila, Mexico, with comparison with adjoining statese. ZooKeys 593: 117–137. doi: 10.3897/zookeys.593.8484 Abstract We compiled a checklist of the amphibians and reptiles of the state of Coahuila, Mexico. The list com- prises 133 species (24 amphibians, 109 reptiles), representing 27 families (9 amphibians, 18 reptiles) and 65 genera (16 amphibians, 49 reptiles). Coahuila has a high richness of lizards in the genus Sceloporus. Coahuila has relatively few state endemics, but has several regional endemics. Overlap in the herpetofauna of Coahuila and bordering states is fairly extensive. Of the 132 species of native amphibians and reptiles, eight are listed as Vulnerable, six as Near Threatened, and six as Endangered in the IUCN Red List. In the SEMARNAT listing, 19 species are Subject to Special Protection, 26 are Threatened, and three are in Danger of Extinction. -

COPPERBELLY WATER SNAKE Nerodia Erythrogaster Neglecta Identification, Status, Ecology, and Conservation in the Midwest
COPPERBELLY WATER SNAKE Nerodia erythrogaster neglecta Identification, Status, Ecology, and Conservation in the Midwest IDENTIFICATION General Appearance Adult copperbellies are uniformly dark brown to black on their dorsal surface, and have an orange, red, or perhaps even yellowish belly, the color of which extends up onto the chin. The dark dorsal may appear as ‘finger- like’ projections of color ‘bleeding’ down from the lateral scales. In some cases, Copperbellies clean up pretty well. Their dark dorsal coloration the dark coloration can contrasts with a yellow or orange belly and throat color. Photo heavily invade the belly by M. Myers. color as dark bands. However, the dark coloration never occurs in half-moon crescents. Copperbellies can be quite large, with adults growing to lengths of 40-50 inches. Juveniles, in contrast to the adults, have a strongly blotched and banded pattern similar to that of other young water snakes. Comparisons Amongst Subspecies The copperbelly is the Midwestern representative subspecies of the “plainbelly” water snake, Nerodia erythrogaster, which ranges throughout the central and southeastern United States. The most similar plainbelly subspecies is the Redbelly Water Snake ( N. e. erythrogaster). However, this is an East Coast form. The Yellowbelly Water Snake (N. e. flavigaster) is the subspecies that is closest to the copperbelly geographically. Yellowbellies and copperbellies co-occur in areas of southern Illinois and western Kentucky, and some intergradation is apparent. As suggested by the name, the belly color of the yellowbelly is more typically yellow rather than orange. The dorsal color of the yellowbelly is more of a light brownish than the dark brown or “black” of the copperbelly. -

Venomous Nonvenomous Snakes of Florida
Venomous and nonvenomous Snakes of Florida PHOTOGRAPHS BY KEVIN ENGE Top to bottom: Black swamp snake; Eastern garter snake; Eastern mud snake; Eastern kingsnake Florida is home to more snakes than any other state in the Southeast – 44 native species and three nonnative species. Since only six species are venomous, and two of those reside only in the northern part of the state, any snake you encounter will most likely be nonvenomous. Florida Fish and Wildlife Conservation Commission MyFWC.com Florida has an abundance of wildlife, Snakes flick their forked tongues to “taste” their surroundings. The tongue of this yellow rat snake including a wide variety of reptiles. takes particles from the air into the Jacobson’s This state has more snakes than organs in the roof of its mouth for identification. any other state in the Southeast – 44 native species and three nonnative species. They are found in every Fhabitat from coastal mangroves and salt marshes to freshwater wetlands and dry uplands. Some species even thrive in residential areas. Anyone in Florida might see a snake wherever they live or travel. Many people are frightened of or repulsed by snakes because of super- stition or folklore. In reality, snakes play an interesting and vital role K in Florida’s complex ecology. Many ENNETH L. species help reduce the populations of rodents and other pests. K Since only six of Florida’s resident RYSKO snake species are venomous and two of them reside only in the northern and reflective and are frequently iri- part of the state, any snake you en- descent. -

Great Dismal Swamp Northern Brown Snake
Snakes Toads and Frogs Brown water snake................................Nerodia taxispilota Eastern spadefoot..............Scaphiopus holbrooki holbrooki U.S. Fish and Wildlife Service Red-bellied water snake...................Nerodia erythrogaster American toad...........................................Bufo americanus erythrogaster Southern toad................................................Bufo terrestris Northern water snake...................Nerodia sipedon sipedon Fowler’s toad................................Bufo woodhousii fowleri Great Dismal Swamp Northern brown snake... ..................Storeria dekayi dekayi Oak toad.......................................................Bufo quercicus Northern red-bellied snake.........Storeria occipitomaculata Spring peeper........................................Pseudacris crucifer National Wildlife Refuge occipitamaculata Pinewoods tree frog......................................Hyla femoralis Eastern ribbon snake.............Thamnophis sauritus sauritus Squirrel tree frog...........................................Hyla squirella Eastern garter snake.................Thamnophis sirtalis sirtalis Gray tree frog..............................................Hyla versicolor Eastern earth snake.....................Virginia valeriae valeriae Little grass frog....................................Pseudacris ocularis Animals of the Eastern hognose snake...Heterodon platirhinos platirhinos Upland chorus frog..............Pseudacris triseriata feriarum Southern ringneck snake....Diadophis punctatus punctatus -

Dorsal and Ventral Color Patterns in a South Georgia Population of Agkistrodon Piscivorus Contanti, the Florida Cottonmouth David L
Georgia Journal of Science Volume 75 No. 2 Scholarly Contributions from the Article 13 Membership and Others 2017 Dorsal and Ventral Color Patterns in a South Georgia Population of Agkistrodon piscivorus contanti, the Florida Cottonmouth David L. Bechler Valdosta State University, [email protected] Joseph A. Kirkley Mr. Wiregrass Georgia Technical College, [email protected] John F. Elder Valdosta State University, [email protected] Follow this and additional works at: http://digitalcommons.gaacademy.org/gjs Part of the Life Sciences Commons Recommended Citation Bechler, David L.; Kirkley, Joseph A. Mr.; and Elder, John F. (2017) "Dorsal and Ventral Color Patterns in a South Georgia Population of Agkistrodon piscivorus contanti, the Florida Cottonmouth," Georgia Journal of Science, Vol. 75, No. 2, Article 13. Available at: http://digitalcommons.gaacademy.org/gjs/vol75/iss2/13 This Research Articles is brought to you for free and open access by Digital Commons @ the Georgia Academy of Science. It has been accepted for inclusion in Georgia Journal of Science by an authorized editor of Digital Commons @ the Georgia Academy of Science. Dorsal and Ventral Color Patterns in a South Georgia Population of Agkistrodon piscivorus contanti, the Florida Cottonmouth Cover Page Footnote As the editor-in-chief of the Georgia Journal of Science, upon submission of this manuscript, I, David L. Bechler, have recused myself of all aspects of the review process and assigned the associate editor all aspects of the review process to include acceptance or rejection. This research articles is available in Georgia Journal of Science: http://digitalcommons.gaacademy.org/gjs/vol75/iss2/13 Bechler et al.: Dorsal and Ventral Agkistrodon piscivorus Coloring Patterns DORSAL AND VENTRAL COLOR PATTERNS IN A SOUTH GEORGIA POPULATION OF Agkistrodon piscivorus conanti, THE FLORIDA COTTONMOUTH 1David L. -

From Plain-Bellied Watersnake, Nerodia Erythrogaster (Serpentes: Colubridae: Natricinae), from Oklahoma Chris T
72 Coccidian from Nerodia erythrogaster First Report of Eimeria hydrophis (Apicomplexa: Eimeriidae) from Plain-Bellied Watersnake, Nerodia erythrogaster (Serpentes: Colubridae: Natricinae), from Oklahoma Chris T. McAllister Science and Mathematics Division, Eastern Oklahoma State College, Idabel, OK 74745 John A. Hnida Department of Microbiology and Immunology, Midwestern University, Glendale, AZ 85308 Abstract: There are several reports of coccidian parasites (Apicomplexa) from plain-bellied watersnakes, Nerodia erythrogaster collected from various locales in Arkansas and Texas. However, there are no reports of coccidians from this host or any other watersnake in Oklahoma. Here, we document, for the first time, Eimeria hydrophis from a N. erythrogaster collected in Oklahoma, as well as provide the first photomicrographs of the coccidian. previously reported from N. erythrogaster (Table 1), including Eimeria attenuata Wacha Introduction and Christiansen, 1974, E. conanti McAllister and Upton, 1989, E. cyclopion McAllister, The plain-bellied watersnake, Nerodia Upton, and Trauth, 1990, an E. helmisophis-like erythrogaster (Forster, 1771) is a large, heavily- coccidian, E. hydrophis Wacha and Christiansen, bodied reptile that ranges from the Delmarva 1974, E. natricis Wacha and Christiansen, 1974, Peninsula south to northern Florida and E. sipedon Wacha and Christiansen, 1975, westward to Oklahoma, New Mexico, Texas, and and E. tenuis Upton and McAllister, 1988 adjacent México; there are isolated populations (Duszynski and Upton 2009; McAllister et al. in the upper Midwest (Powell et al. 2016). In 2017). Here, we document a new geographic Oklahoma, this snake is found statewide (Sievert record for E. hydrophis from a N. erythrogaster and Sievert 2011). It inhabits a wide variety of collected from Oklahoma, and provide the first wetlands, but mostly larger, more permanent photomicrographs of the coccidian.