Piperazine Derivatives As Dangerous Abused Compounds
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Identifying New/Emerging Psychoactive Substances at the Time of COVID-19; a Web-Based Approach
ORIGINAL RESEARCH published: 09 February 2021 doi: 10.3389/fpsyt.2020.632405 Identifying New/Emerging Psychoactive Substances at the Time of COVID-19; A Web-Based Approach Valeria Catalani 1*, Davide Arillotta 1, John Martin Corkery 1, Amira Guirguis 1,2, Alessandro Vento 3,4,5 and Fabrizio Schifano 1 1 Psychopharmacology, Drug Misuse & Novel Psychoactive Substances Research Unit, School of Life & Medical Sciences, University of Hertfordshire, Hatfield, United Kingdom, 2 Swansea University Medical School, Institute of Life Sciences 2, Swansea University, Swansea, United Kingdom, 3 Department of Mental Health, ASL Roma 2, Rome, Italy, 4 Addictions’ Observatory (ODDPSS), Rome, Italy, 5 Department of Psychology, Guglielmo Marconi University, Rome, Italy COVID-19-related disruptions of people and goods’ circulation can affect drug markets, especially for new psychoactive substances (NPSs). Drug shortages could cause a change in available NPS, with the introduction of new, unknown, substances. The aims of the current research were to use a web crawler, NPSfinder®, to identify and categorize emerging NPS discussed on a range of drug enthusiasts/psychonauts’ websites/fora at the time of the pandemic; social media for these identified NPS were screened as Edited by: well. The NPSfinder® was used here to automatically scan 24/7 a list of psychonaut Ornella Corazza, University of Hertfordshire, websites and NPS online resources. The NPSs identified in the time frame between United Kingdom January and August 2020 were searched in both the European Monitoring Center Reviewed by: for Drugs and Drug Addictions (EMCDDA)/United Nations Office on Drugs and Crime Simona Zaami, Sapienza University of Rome, Italy (UNODC) databases and on social media (Facebook, Twitter, Instagram, Pinterest, Laura Hondebrink, and YouTube) as well, with a content qualitative analysis having been carried out on University Medical Center reddit.com. -

Substance Abuse: the Pharmacy Educator’S Role in Prevention and Recovery
Substance Abuse: The Pharmacy Educator’s Role in Prevention and Recovery Curricular Guidelines for Pharmacy: Substance Abuse and Addictive Disease i Curricular Guidelines for Pharmacy: Substance Abuse and Addictive Disease1,2 BACKGROUND OF THE CURRICULUM DEVELOPMENT PROJECT In 1988, the AACP Special Interest Group (SIG) on Pharmacy Student and Faculty Impairment (renamed Substance Abuse Education and Assistance) undertook the development of curricular guidelines for colleges/schools of pharmacy to facilitate the growth of educational opportunities for student pharmacists. These Curricular Guidelines for Pharmacy Education: Substance Abuse and Addictive Disease were published in 1991 (AJPE. 55:311-16. Winter 1991.) One of the charges of the Special Committee on Substance Abuse and Pharmacy Education was to review and revise the 1991 curricular guidelines. Overall, the didactic and experiential components in the suggested curriculum should prepare the student pharmacist to competently problem-solve issues concerning alcohol and other drug abuse and addictive diseases affecting patients, families, colleagues, themselves, and society. The guidelines provide ten educational goals, while describing four major content areas including: psychosocial aspects of alcohol and other drug use; pharmacology and toxicology of abused substances; identification, intervention, and treatment of people with addictive diseases; and legal/ethical issues. The required curriculum suggested by these guidelines addresses the 1 These guidelines were revised by the AACP Special Committee on Substance Abuse and Pharmacy Education. Members drafting the revised guidelines were Edward M. DeSimone (Creighton University), Julie C. Kissack (Harding University), David M. Scott (North Dakota State University), and Brandon J. Patterson (University of Iowa). Other Committee members were Paul W. Jungnickel, Chair (Auburn University), Lisa A. -

(19) United States (12) Patent Application Publication (10) Pub
US 20130289061A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2013/0289061 A1 Bhide et al. (43) Pub. Date: Oct. 31, 2013 (54) METHODS AND COMPOSITIONS TO Publication Classi?cation PREVENT ADDICTION (51) Int. Cl. (71) Applicant: The General Hospital Corporation, A61K 31/485 (2006-01) Boston’ MA (Us) A61K 31/4458 (2006.01) (52) U.S. Cl. (72) Inventors: Pradeep G. Bhide; Peabody, MA (US); CPC """"" " A61K31/485 (201301); ‘4161223011? Jmm‘“ Zhu’ Ansm’ MA. (Us); USPC ......... .. 514/282; 514/317; 514/654; 514/618; Thomas J. Spencer; Carhsle; MA (US); 514/279 Joseph Biederman; Brookline; MA (Us) (57) ABSTRACT Disclosed herein is a method of reducing or preventing the development of aversion to a CNS stimulant in a subject (21) App1_ NO_; 13/924,815 comprising; administering a therapeutic amount of the neu rological stimulant and administering an antagonist of the kappa opioid receptor; to thereby reduce or prevent the devel - . opment of aversion to the CNS stimulant in the subject. Also (22) Flled' Jun‘ 24’ 2013 disclosed is a method of reducing or preventing the develop ment of addiction to a CNS stimulant in a subj ect; comprising; _ _ administering the CNS stimulant and administering a mu Related U‘s‘ Apphcatlon Data opioid receptor antagonist to thereby reduce or prevent the (63) Continuation of application NO 13/389,959, ?led on development of addiction to the CNS stimulant in the subject. Apt 27’ 2012’ ?led as application NO_ PCT/US2010/ Also disclosed are pharmaceutical compositions comprising 045486 on Aug' 13 2010' a central nervous system stimulant and an opioid receptor ’ antagonist. -

Programme & Abstracts
The 57th Annual Meeting of the International Association of Forensic Toxicologists. 2nd - 6th September 2019 BIRMINGHAM, UK The ICC Birmingham Broad Street, Birmingham B1 2EA Programme & Abstracts 1 Thank You to our Sponsors PlatinUm Gold Silver Bronze 2 3 Contents Welcome message 5 Committees 6 General information 7 iCC maps 8 exhibitors list 10 Exhibition Hall 11 Social Programme 14 opening Ceremony 15 Schedule 16 Oral Programme MONDAY 2 September 19 TUESDAY 3 September 21 THURSDAY 5 September 28 FRIDAY 6 September 35 vendor Seminars 42 Posters 46 oral abstracts 82 Poster abstracts 178 4 Welcome Message It is our great pleasure to welcome you to TIAFT Gala Dinner at the ICC on Friday evening. On the accompanying pages you will see a strong the UK for the 57th Annual Meeting of scientific agenda relevant to modern toxicology and we The International Association of Forensic thank all those who submitted an abstract and the Toxicologists Scientific Committees for making the scientific programme (TIAFT) between 2nd and 6th a success. Starting with a large Young Scientists September 2019. Symposium and Dr Yoo Memorial plenary lecture by Prof Tony Moffat on Monday, there are oral session topics in It has been decades since the Annual Meeting has taken Clinical & Post-Mortem Toxicology on Tuesday, place in the country where TIAFT was founded over 50 years Human Behaviour Toxicology & Drug-Facilitated Crime on ago. The meeting is supported by LTG (London Toxicology Thursday and Toxicology in Sport, New Innovations and Group) and the UKIAFT (UK & Ireland Association of Novel Research & Employment/Occupational Toxicology Forensic Toxicologists) and we thank all our exhibitors and on Friday. -

Ecstasy Or Molly, Is a Synthetic Psychoactive Drug
DRUG ENFORCEMENT ADMINISTRATION THE FACTS ABOUT MDMA Ecstasy &Molly DEA PHOTO What is it? MDMA, (3,4-methylenedioxy- methamphetamine), known as Ecstasy or Molly, is a synthetic psychoactive drug. Ecstasy is the pill form of MDMA. Molly is the slang for “molecular” that refers to the powder or crystal form of MDMA. Molly is often mixed with other drugs and substances and is not pure MDMA or safe to use. How is it used? MDMA or Ecstasy is taken orally in pill or tablet form. These pills can be in different colors with images on them. Molly is taken in a gel capsule or snorted. What does MDMA do to the body and mind? • As a stimulant drug, it increases heart rate and blood pressure. Users may experience muscle tension, involuntary teeth clenching, nausea, blurred vision, faintness, chills, or sweating • It produces feelings of increased energy, euphoria, emotional warmth, empathy, and distortions in sensory and time perception. • Feelings of sadness, anxiety, depression and memory difficulties are other effects. • It can seriously deplete serotonin levels in the brain, causing confusion and sleep problems. Did you know? • DEA has labeled MDMA as a Schedule I drug, meaning its abuse potential is high and it has no approved medical use. It is illegal in the U.S. • In high doses, MDMA can affect the body’s ability to regulate temperature, which can lead to serious health complications and possible death. • Teens are using less MDMA. Teens decreased their past year use of MDMA from 1.9% in 2010 to 1.2% of teens using 2012. -

WO 2015/072852 Al 21 May 2015 (21.05.2015) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2015/072852 Al 21 May 2015 (21.05.2015) P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A61K 36/84 (2006.01) A61K 31/5513 (2006.01) kind of national protection available): AE, AG, AL, AM, A61K 31/045 (2006.01) A61P 31/22 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, A61K 31/522 (2006.01) A61K 45/06 (2006.01) BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (21) International Application Number: HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, PCT/NL20 14/050780 KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, (22) International Filing Date: MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, 13 November 2014 (13.1 1.2014) PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, (25) Filing Language: English TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (26) Publication Language: English (84) Designated States (unless otherwise indicated, for every (30) Priority Data: kind of regional protection available): ARIPO (BW, GH, 61/903,430 13 November 2013 (13. 11.2013) US GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, (71) Applicant: RJG DEVELOPMENTS B.V. -
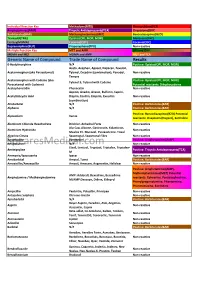
Cross Reaction Guide
Individual Reaction Key Methadone(MTD) Phencyclidine(PCP) Amphetamines(AMP) Tricyclic Antidepressants(TCA) Oxycodone(OXY) Barbiturates(BAR) Methamphetamines(MET) Benzodiazepines(BZO) Tramadol(TML) Opiates(OPI, MOP, MOR) Marijuana(THC) Ecstasy(MDMA) Cotinine(COT) Cocaine(COC) Buprenorphine(BUP) Propoxyphene(PPX) Non-reactive Multiple Reaction Key MET and AMP OPI and OXY MDMA and MET MDMA and AMP MET and TCA Generic Name of Compound Trade Name of Compound Results 6-Acetylmorphine N/A Positive: Opiates(OPI, MOP, MOR) Aceta, Acephen, Apacet, Dapacen, Feverall, Acetaminophen (aka Paracetamol) Tylenol, Excedrin (combination), Panadol, Non-reactive Tempra Acetaminophen with Codeine (aka Positive: Opiates(OPI, MOP, MOR) Tylenol 3, Tylenol with Codeine Paracetamol with Codeine) Potential reactants: Dihydrocodeine Acetophenetidin Phenacetin Non-reactive Aspirin, Anadin, Anasin, Bufferin, Caprin, Acetylsalicyclic Acid Disprin, Ecotrin, Empirin, Excedrin Non-reactive (combination) Allobarbital N/A Positive: Barbiturates(BAR) Alphenol N/A Positive: Barbiturates(BAR) Positive: Benzodiazepines(BZO) Potential Alprazolam Xanax reactants: Oxaprozin (Daypro), Sertraline Aluminum Chloride Hexahydrate Drichlor, Anhydrol Forte Non-reactive Alu-Cap, Alisone, Gastrocote, Kolanticon, Aluminum Hydroxide Non-reactive Maalox TC, Mucogel, Pyrogastrone, Topal Alverine Citrate Spasmonal, Spasmonal Fibre Non-reactive Amantadine Symmetrel Positive: Amphetamines(AMP) SpearesMedical.comAminopyrine N/A Non-reactive Elavil, Lentizol, Tryptizol, Triptafen, Triptafen- Amitriptyline -

References to Argentina
References to Argentina Part 1 RECENT STATISTICS AND TREND ANALYSIS OF ILLICIT DRUG MARKETS A. EXTENT OF ILLICIT DRUG USE AND HEALTH CONSEQUENCES The Americas (pages 12 to 14 WDR 13) In the Americas, a high prevalence of most illicit drugs, essentially driven by estimates in North America, was observed, with the prevalence of cannabis (7.9 per cent) and cocaine (1.3 per cent) being particularly high in the region. South America, Central America and the Caribbean The annual prevalence of cocaine use in South America (1.3 per cent of the adult population) is comparable to levels in North America, while it remains much higher than the global average in Central America (0.6 per cent) and the Caribbean (0.7 per cent). Cocaine use has increased significantly in Brazil, Costa Rica and, to lesser extent, Peru while no change in its use was reported in Argentina. The use of cannabis in South America is higher (5.7 per cent) than the global average, but lower in Central America and Caribbean (2.6 and 2.8 per cent respectively). In South America and Central America the use of opioids (0.3 and 0.2 per cent, respectively) and Ecstasy (0.1 per cent each) also remain well below the global average. While opiates use remains low, countries such as Colombia report that heroin use is becoming increasingly common among certain age groups and socio-economic classes.30 Part 2 NEW PSYCHOACTIVE SUBSTANCES C. THE RECENT EMERGENCE AND SPREAD OF NEW PSYCHOACTIVE SUBSTANCES (page 67 WDR 13) Spread at the global level Number of countries reporting the emergence of new psychoactive substances Pursuant to Commission on Narcotic Drugs resolution 55/1, entitled “Promoting international cooperation in responding to the challenges posed by new psychoactive substances”, in 2012 UNODC sent a questionnaire on NPS to all Member States, to which 80 countries and territories replied. -

Piperazine (Street Names: “TFMPP” Or “Molly”. Often Found in Combination with BZP: “A2”, “Legal E” Or “Legal X”)
Drug Enforcement Administration Diversion Control Division Drug & Chemical Evaluation Section 1-[3-(Trifluoro-methyl)-phenyl]piperazine (Street Names: “TFMPP” or “Molly”. Often found in combination with BZP: “A2”, “Legal E” or “Legal X”) October 2019 Introduction: Illicit Uses: 1-[3-(Trifluoro-methyl)-phenyl]piperazine (TFMPP) is TFMPP is being promoted as a legal alternative to an industrial chemical. It is often abused in combination MDMA at raves (all-night dance parties) as TFMPP or with benzylpiperazine (BZP), a schedule I controlled “Molly” and is often sold in combination with BZP as substance. The Drug Enforcement Administration (DEA) “ecstasy”, or “A2", “legal E” or “legal X” in order to enhance temporarily controlled TFMPP in 2002 as a schedule I its spectrum of effects. TFMPP may be abused alone for hallucinogen under the Controlled Substances Act (CSA) its hallucinogenic effects. TFMPP is generally administered because of its abuse potential and lack of accepted orally as either powder, tablets or capsules. Other routes medical use or safety. However, based on the scientific of administration include smoking and snorting. and medical evaluation conducted by the Food and Drug Administration (FDA) and the National Institute on Drug User Population: Abuse (NIDA), the Department of Health and Human Youth and young adults are the main abusers of Services (DHHS) did not recommend control of TFMPP. TFMPP. Accordingly, TFMPP was no longer controlled under the CSA after March 18, 2004. Recently, there has been an Illicit Distribution: escalation in the abuse of TFMPP in the United States as The National Forensic Laboratory Information System evidenced by the increasing encounters of this substance (NFLIS) is a DEA database that collects scientifically by law enforcement officials in various states and the verified data on drug items and cases submitted to and District of Columbia. -

Ce4less.Com Ce4less.Com Ce4less.Com Ce4less.Com Ce4less.Com Ce4less.Com Ce4less.Com
Hallucinogens And Dissociative Drug Use And Addiction Introduction Hallucinogens are a diverse group of drugs that cause alterations in perception, thought, or mood. This heterogeneous group has compounds with different chemical structures, different mechanisms of action, and different adverse effects. Despite their description, most hallucinogens do not consistently cause hallucinations. The drugs are more likely to cause changes in mood or in thought than actual hallucinations. Hallucinogenic substances that form naturally have been used worldwide for millennia to induce altered states for religious or spiritual purposes. While these practices still exist, the more common use of hallucinogens today involves the recreational use of synthetic hallucinogens. Hallucinogen And Dissociative Drug Toxicity Hallucinogens comprise a collection of compounds that are used to induce hallucinations or alterations of consciousness. Hallucinogens are drugs that cause alteration of visual, auditory, or tactile perceptions; they are also referred to as a class of drugs that cause alteration of thought and emotion. Hallucinogens disrupt a person’s ability to think and communicate effectively. Hallucinations are defined as false sensations that have no basis in reality: The sensory experience is not actually there. The term “hallucinogen” is slightly misleading because hallucinogens do not consistently cause hallucinations. 1 ce4less.com ce4less.com ce4less.com ce4less.com ce4less.com ce4less.com ce4less.com How hallucinogens cause alterations in a person’s sensory experience is not entirely understood. Hallucinogens work, at least in part, by disrupting communication between neurotransmitter systems throughout the body including those that regulate sleep, hunger, sexual behavior and muscle control. Patients under the influence of hallucinogens may show a wide range of unusual and often sudden, volatile behaviors with the potential to rapidly fluctuate from a relaxed, euphoric state to one of extreme agitation and aggression. -

Piperazine (MDBZP)
1-(3-4- methylendioxybenzyl)piperazine (MDBZP) Pre-Review Report Expert Committee on Drug Dependence Thirty-fifth Meeting Hammamet, Tunisia, 4-8 June 2012 35th ECDD (2012) Agenda item 5.3e 1-(3-4-methylendioxybenzyl)piperazine (MDBZP) - 2 - 35th ECDD (2012) Agenda item 5.3e 1-(3-4-methylendioxybenzyl)piperazine (MDBZP) Acknowledgements This report has been drafted under the responsibility of the WHO Secretariat, Essential Medicines and Health Products, Medicines Access and Rational Use Unit. The WHO Secretariat would like to thank the following people for their contribution in producing this pre-review report: Dr Simon Elliott, United Kingdom (literature review and drafting), Dr Caroline Bodenschatz (editing) and Mr Kamber Celebi, France (questionnaire report). - 3 - 35th ECDD (2012) Agenda item 5.3e 1-(3-4-methylendioxybenzyl)piperazine (MDBZP) - 4 - 35th ECDD (2012) Agenda item 5.3e 1-(3-4-methylendioxybenzyl)piperazine (MDBZP) Contents SUMMARY ............................................................................................................................... 7 1. Substance identification .................................................................................................... 8 A. International Nonproprietary Name (INN) ................................................................ 8 B. Chemical Abstract Service (CAS) Registry Number ................................................. 8 C. Other Names .............................................................................................................. -

Association of Selective Serotonin Reuptake Inhibitors with the Risk for Spontaneous Intracranial Hemorrhage
Supplementary Online Content Renoux C, Vahey S, Dell’Aniello S, Boivin J-F. Association of selective serotonin reuptake inhibitors with the risk for spontaneous intracranial hemorrhage. JAMA Neurol. Published online December 5, 2016. doi:10.1001/jamaneurol.2016.4529 eMethods 1. List of Antidepressants for Cohort Entry eMethods 2. List of Antidepressants According to the Degree of Serotonin Reuptake Inhibition eMethods 3. Potential Confounding Variables Included in Multivariate Models eMethods 4. Sensitivity Analyses eFigure. Flowchart of Incident Antidepressant (AD) Cohort Definition and Case- Control Selection eTable 1. Crude and Adjusted Rate Ratios of Intracerebral Hemorrhage Associated With Current Use of SSRIs Relative to TCAs eTable 2. Crude and Adjusted Rate Ratios of Subarachnoid Hemorrhage Associated With Current Use of SSRIs Relative to TCAs eTable 3. Crude and Adjusted Rate Ratios of Intracranial Extracerebral Hemorrhage Associated With Current Use of SSRIs Relative to TCAs. eTable 4. Crude and Adjusted Rate Ratios of Intracerebral Hemorrhage Associated With Current Use of Antidepressants With Strong Degree of Inhibition of Serotonin Reuptake Relative to Weak eTable 5. Crude and Adjusted Rate Ratios of Subarachnoid Hemorrhage Associated With Current Use of Antidepressants With Strong Degree of Inhibition of Serotonin Reuptake Relative to Weak eTable 6. Crude and Adjusted Rate Ratios of Intracranial Extracerebral Hemorrhage Associated With Current Use of Antidepressants With Strong Degree of Inhibition of Serotonin Reuptake Relative to Weak This supplementary material has been provided by the authors to give readers additional information about their work. © 2016 American Medical Association. All rights reserved. Downloaded From: https://jamanetwork.com/ on 10/02/2021 eMethods 1.