Elucidation of Antimicrobial Activity and Mechanism of Action by N-Substituted Carbazole Derivatives
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Plasma Membrane Ca2+–Atpase in Rat and Human Odontoblasts Mediates Dentin Mineralization
biomolecules Article Plasma Membrane Ca2+–ATPase in Rat and Human Odontoblasts Mediates Dentin Mineralization Maki Kimura 1,†, Hiroyuki Mochizuki 1,†, Ryouichi Satou 2, Miyu Iwasaki 2, Eitoyo Kokubu 3, Kyosuke Kono 1, Sachie Nomura 1, Takeshi Sakurai 1, Hidetaka Kuroda 1,4,† and Yoshiyuki Shibukawa 1,*,† 1 Department of Physiology, Tokyo Dental College, 2-9-18, Kanda-Misaki-cho, Chiyoda-ku, Tokyo 101-0061, Japan; [email protected] (M.K.); [email protected] (H.M.); [email protected] (K.K.); [email protected] (S.N.); [email protected] (T.S.); [email protected] (H.K.) 2 Department of Epidemiology and Public Health, Tokyo Dental College, Chiyodaku, Tokyo 101-0061, Japan; [email protected] (R.S.); [email protected] (M.I.) 3 Department of Microbiology, Tokyo Dental College, Chiyodaku, Tokyo 101-0061, Japan; [email protected] 4 Department of Dental Anesthesiology, Kanagawa Dental University, 1-23, Ogawacho, Kanagawa, Yokosuka-shi 238-8570, Japan * Correspondence: [email protected] † These authors contributed equally to this study. Abstract: Intracellular Ca2+ signaling engendered by Ca2+ influx and mobilization in odontoblasts is critical for dentinogenesis induced by multiple stimuli at the dentin surface. Increased Ca2+ is exported by the Na+–Ca2+ exchanger (NCX) and plasma membrane Ca2+–ATPase (PMCA) to Citation: Kimura, M.; Mochizuki, H.; maintain Ca2+ homeostasis. We previously demonstrated a functional coupling between Ca2+ Satou, R.; Iwasaki, M.; Kokubu, E.; extrusion by NCX and its influx through transient receptor potential channels in odontoblasts. Kono, K.; Nomura, S.; Sakurai, T.; Although the presence of PMCA in odontoblasts has been previously described, steady-state levels of Kuroda, H.; Shibukawa, Y. -

SERCA in Genesis of Arrhythmias: What We Already Know and What Is New?
Review 43 SERCA in genesis of arrhythmias: what we already know and what is new? Nilüfer Erkasap Department of Physiology, Medical Faculty, Eskiflehir Osmangazi University, Eskiflehir, Turkey ABSTRACT This review mainly focuses on the structure, function of the sarco(endo)plasmic reticulum calcium pump (SERCA) and its role in genesis of arrhythmias. SERCA is a membrane protein that belongs to the family of P-type ion translocating ATPases and pumps free cytosolic calcium into intracellular stores. Active transport of Ca2+ is achieved, according to the E1-E2 model, changing of SERCA structure by Ca2+. The affinity of Ca2+ -binding sites varies from high (E1) to low (E2). Three different SERCA genes were identified-SERCA1, SERCA2, and SERCA3. SERCA is mainly represented by the SERCA2a isoform in the heart. In heart muscle, during systole, depolarization triggers the release of Ca2+ from the sarcoplasmic reticulum (SR) and starts contraction. During diastole, muscle relaxation occurs as Ca2+ is again removed from cytosol, predominantly by accumulation into SR via the action of SERCA2a. The main regulator of SERCA2a is phospholamban and another regulator proteolipid of SERCA is sarcolipin. There are a lot of studies on the effect of decreased and/or increased SERCA activity in genesis of arrhythmia. Actually both decrease and increase of SERCA activity in the heart result in some pathological mechanisms such as heart failure and arrhythmia. (Anadolu Kardiyol Derg 2007: 7 Suppl 1; 43-6) Key words: sarco(endo)plasmic reticulum, SERCA, arrhythmia, calcium channels Introduction from cytosol, predominantly by accumulation into sarcoplasmic reticulum via the action of sarco(endo)plasmic reticulum Cardiac physiology is a major area of research in basic and Ca ATPase (SERCA). -

Endoplasmic Reticulum Potassium–Hydrogen Exchanger and Small
Research Article 625 Endoplasmic reticulum potassium–hydrogen exchanger and small conductance calcium-activated potassium channel activities are essential for ER calcium uptake in neurons and cardiomyocytes Malle Kuum1,2,3, Vladimir Veksler2,3, Joanna Liiv1, Renee Ventura-Clapier2,3 and Allen Kaasik1,* 1Department of Pharmacology, Centre of Excellence for Translational Medicine, University of Tartu, Ravila 19, Tartu EE-51014, Estonia 2INSERM, U-769, 5, rue Jean-Baptiste Clement, Chaˆtenay-Malabry F-92296, France 3Universite´ Paris-Sud, 5, rue Jean-Baptiste Clement, Chaˆtenay-Malabry F-92296, France *Author for correspondence ([email protected]) Accepted 12 September 2011 Journal of Cell Science 125, 625–633 ß 2012. Published by The Company of Biologists Ltd doi: 10.1242/jcs.090126 Summary Calcium pumping into the endoplasmic reticulum (ER) lumen is thought to be coupled to a countertransport of protons through sarcoplasmic/endoplasmic reticulum calcium ATPase (SERCA) and the members of the ClC family of chloride channels. However, pH in the ER lumen remains neutral, which suggests a mechanism responsible for proton re-entry. We studied whether cation–proton exchangers could act as routes for such a re-entry. ER Ca2+ uptake was measured in permeabilized immortalized hypothalamic neurons, primary rat cortical neurons and mouse cardiac fibers. Replacement of K+ in the uptake solution with Na+ or tetraethylammonium led to a strong inhibition of Ca2+ uptake in neurons and cardiomyocytes. Furthermore, inhibitors of the potassium–proton exchanger (quinine or propranolol) but not of the sodium–proton exchanger reduced ER Ca2+ uptake by 56–82%. Externally added nigericin, a potassium– + proton exchanger, attenuated the inhibitory effect of propranolol. -

Crystal Structure of the Calcium Pump of Sarcoplasmic Reticulum at 2.6 AÊ Resolution
articles Crystal structure of the calcium pump of sarcoplasmic reticulum at 2.6 AÊ resolution Chikashi Toyoshima*², Masayoshi Nakasako*²³, Hiromi Nomura* & Haruo Ogawa* * Institute of Molecular and Cellular Biosciences, The University of Tokyo, Bunkyo-ku, Tokyo 113-0032, Japan ² The Harima Institute, The Institute of Physical and Chemical Research, Sayo-gun, Hyo-go 679-5143, Japan ³ PRESTO, Japan Science and Technology Corporation, Kawaguchi 332-0012, Japan ............................................................................................................................................................................................................................................................................ Calcium ATPase is a member of the P-type ATPases that transport ions across the membrane against a concentration gradient. Here we have solved the crystal structure of the calcium ATPase of skeletal muscle sarcoplasmic reticulum (SERCA1a) at 2.6 AÊ resolution with two calcium ions bound in the transmembrane domain, which comprises ten a-helices. The two calcium ions are located side by side and are surrounded by four transmembrane helices, two of which are unwound for ef®cient coordination geometry. The cytoplasmic region consists of three well separated domains, with the phosphorylation site in the central catalytic domain and the adenosine-binding site on another domain. The phosphorylation domain has the same fold as haloacid dehalogenase. Comparison with a low-resolution electron density map of the enzyme in the absence -

Clinical Significance of P‑Class Pumps in Cancer (Review)
ONCOLOGY LETTERS 22: 658, 2021 Clinical significance of P‑class pumps in cancer (Review) SOPHIA C. THEMISTOCLEOUS1*, ANDREAS YIALLOURIS1*, CONSTANTINOS TSIOUTIS1, APOSTOLOS ZARAVINOS2,3, ELIZABETH O. JOHNSON1 and IOANNIS PATRIKIOS1 1Department of Medicine, School of Medicine; 2Department of Life Sciences, School of Sciences, European University Cyprus, 2404 Nicosia, Cyprus; 3College of Medicine, Member of Qatar University Health, Qatar University, 2713 Doha, Qatar Received January 25, 2021; Accepted Apri 12, 2021 DOI: 10.3892/ol.2021.12919 Abstract. P‑class pumps are specific ion transporters involved Contents in maintaining intracellular/extracellular ion homeostasis, gene transcription, and cell proliferation and migration in all 1. Introduction eukaryotic cells. The present review aimed to evaluate the 2. Methodology role of P‑type pumps [Na+/K+ ATPase (NKA), H+/K+ ATPase 3. NKA (HKA) and Ca2+‑ATPase] in cancer cells across three fronts, 4. SERCA pump namely structure, function and genetic expression. It has 5. HKA been shown that administration of specific P‑class pumps 6. Clinical studies of P‑class pump modulators inhibitors can have different effects by: i) Altering pump func‑ 7. Concluding remarks and future perspectives tion; ii) inhibiting cell proliferation; iii) inducing apoptosis; iv) modifying metabolic pathways; and v) induce sensitivity to chemotherapy and lead to antitumor effects. For example, 1. Introduction the NKA β2 subunit can be downregulated by gemcitabine, resulting in increased apoptosis of cancer cells. The sarco‑ The movement of ions across a biological membrane is a endoplasmic reticulum calcium ATPase can be inhibited by crucial physiological process necessary for maintaining thapsigargin resulting in decreased prostate tumor volume, cellular homeostasis. -

Plasma Membrane Ca2+ Atpase Isoform 4 (PMCA4) Has an Important Role in Numerous Hallmarks of Pancreatic Cancer
cancers Article Plasma Membrane Ca2+ ATPase Isoform 4 (PMCA4) Has an Important Role in Numerous Hallmarks of Pancreatic Cancer Pishyaporn Sritangos 1 , Eduardo Pena Alarcon 1, Andrew D. James 2, Ahlam Sultan 3, Daniel A. Richardson 1 and Jason I. E. Bruce 1,* 1 Division of Cancer Sciences, School of Medical Sciences, Faculty of Biology, Medicine and Health, University of Manchester, Manchester M13 9PT, UK; [email protected] (P.S.); [email protected] (E.P.A.); [email protected] (D.A.R.) 2 Department of Biology, University of York, Heslington, York YO10 5DD, UK; [email protected] 3 Department of Pharmaceutical Science, College of Pharmacy, Princess Nourah Bint Abdulrahman University, Riyadh, Saudi Arabia; [email protected] * Correspondence: [email protected]; Tel.:+44-16-12-75-54-84 Received: 13 November 2019; Accepted: 10 January 2020; Published: 16 January 2020 Abstract: Pancreatic ductal adenocarcinoma (PDAC) is largely resistant to standard treatments leading to poor patient survival. The expression of plasma membrane calcium ATPase-4 (PMCA4) is reported to modulate key cancer hallmarks including cell migration, growth, and apoptotic resistance. Data-mining revealed that PMCA4 was over-expressed in pancreatic ductal adenocarcinoma (PDAC) tumors which correlated with poor patient survival. Western blot and RT-qPCR revealed that MIA PaCa-2 cells almost exclusively express PMCA4 making these a suitable cellular model of PDAC with poor patient survival. Knockdown of PMCA4 in MIA PaCa-2 cells (using siRNA) reduced cytosolic 2+ 2+ Ca ([Ca ]i) clearance, cell migration, and sensitized cells to apoptosis, without affecting cell growth. -

Atpase in the Rabbit Lens
Investigative Ophthalmology & Visual Science, Vol. 30, No. 7, July 1989 Copyright © Association for Research in Vision and Ophthalmology Oxidative Inhibition of Ca2+-ATPase in the Rabbit Lens Douglas Dorchmon, Christopher A. Parerson, and Nicholas A. Delamere Hydrogen peroxide inhibition of maximum Ca2+-ATPase and Na+,K+-ATPase activity was measured in a membrane-enriched preparation of rabbit lens cortical fibers and epithelium. At 5 X 1O~6 M hydrogen peroxide maximum Ca2+-ATPase activity was inhibited by 39%, while maximum Na+,K+- ATPase activity was stimulated. Ca2+-ATPase activity was almost completely inhibited at 5 X 10~4 M hydrogen peroxide, in comparison to Na+,K+-ATPase activity, which was only inhibited by 28% at a concentration of hydrogen peroxide an order of magnitude larger. The addition of catalase to hydrogen peroxide-pretreated samples did not reverse the inhibition of Ca2+-ATPase by hydrogen peroxide. Invest Ophthalmol Vis Sci 30:1633-1637,1989 The possible involvement of lenticular calcium Materials and Methods metabolism in the development of experimental and human cataract has been explored in several stud- Animal Tissues 1 3 ies. " Regulation of lens calcium content is accom- Rabbit eyes were obtained from healthy 2 kg New plished by restricted membrane permeability and a Zealand strain albino rabbits, about 10 weeks old, calcium pump resident in the lens cell membrane. killed painlessly by intravenous administration of 2+ The activity and distribution of Ca -ATPase in the T-61 euthanasia solution (American Hoechst Corp., 4 lens has been described by Hightower et al and Somerville, NJ). Lenses were dissected from the globe Borchman et al.5 by a posterior approach. -

Primary Active Ca2+ Transport Systems in Health and Disease
Downloaded from http://cshperspectives.cshlp.org/ on September 27, 2021 - Published by Cold Spring Harbor Laboratory Press Primary Active Ca2+ Transport Systems in Health and Disease Jialin Chen,1 Aljona Sitsel,1 Veronick Benoy,1 M. Rosario Sepúlveda,2,3 and Peter Vangheluwe1,3 1Laboratory of Cellular Transport Systems, Department of Cellular and Molecular Medicine, KU Leuven, 3000 Leuven, Belgium 2Department of Cell Biology, Faculty of Sciences, University of Granada, 18071 Granada, Spain Correspondence: [email protected] Calcium ions (Ca2+) are prominent cell signaling effectors that regulate a wide variety of cellular processes. Among the different players in Ca2+ homeostasis, primary active Ca2+ transporters are responsible for keeping low basal Ca2+ levels in the cytosol while establishing steep Ca2+ gradients across intracellular membranes or the plasma membrane. This review summarizes our current knowledge on the three types of primary active Ca2+-ATPases: the sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA) pumps, the secretory pathway Ca2+- ATPase (SPCA) isoforms, and the plasma membrane Ca2+-ATPase (PMCA) Ca2+-transporters. We first discuss the Ca2+ transport mechanism of SERCA1a, which serves as a reference to describe the Ca2+ transport of other Ca2+ pumps. We further highlight the common and unique features of each isoform and review their structure–function relationship, expression pattern, regulatory mechanisms, and specific physiological roles. Finally, we discuss the increasing genetic and in vivo evidence that links the dysfunction of specific Ca2+-ATPase isoforms to a broad range of human pathologies, and highlight emerging therapeutic strate- gies that target Ca2+ pumps. a2+ signaling is crucial for many physiolog- cus on the primary active Ca2+-transporters or Cical processes and is dysregulated in a mul- Ca2+-ATPases, which are responsible for keep- titude of pathological conditions. -

Sarcolipin Alters Serca1a Interdomain Communication By
www.nature.com/scientificreports OPEN Sarcolipin alters SERCA1a interdomain communication by impairing binding of both calcium and ATP Cédric Montigny1*, Dong Liang Huang3, Veronica Beswick1,2, Thomas Barbot1, Christine Jaxel1, Marc le Maire1, Ji‑Shen Zheng3* & Nadège Jamin1 Sarcolipin (SLN), a single‑spanning membrane protein, is a regulator of the sarco‑endoplasmic reticulum Ca2+‑ATPase (SERCA1a). Chemically synthesized SLN, palmitoylated or not (pSLN or SLN), and recombinant wild‑type rabbit SERCA1a expressed in S. cerevisiae design experimental conditions that provide a deeper understanding of the functional role of SLN on the regulation of SERCA1a. Our data show that chemically synthesized SLN interacts with recombinant SERCA1a, with calcium‑ deprived E2 state as well as with calcium‑bound E1 state. This interaction hampers the binding of calcium in agreement with published data. Unexpectedly, SLN has also an allosteric efect on SERCA1a transport activity by impairing the binding of ATP. Our results reveal that SLN signifcantly slows down the E2 to Ca2.E1 transition of SERCA1a while it afects neither phosphorylation nor dephosphorylation. Comparison with chemically synthesized SLN deprived of acylation demonstrates that palmitoylation is not necessary for either inhibition or association with SERCA1a. However, it has a small but statistically signifcant efect on SERCA1a phosphorylation when various ratios of SLN‑ SERCA1a or pSLN‑SERCA1a are tested. Abbreviations C12E8 Octaethylene-glycol-dodecylether DDM β-Dodecyl maltoside PLB Phospholamban SLN Sarcolipin PLM Phospholemman SR Sarco-endoplasmic reticulum SERCA1a Sarco-endoplasmic reticulum Ca2+-ATPase, isoform 1a recS1a Recombinant yeast expressed SERCA1a EGTA Ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid Tg Tapsigargin pSLN palmitoylated Sarcolipin Te sarco-endoplasmic reticulum Ca 2+-ATPase (SERCA1a) is a 110 kDa integral membrane transporter and is one of the major actor of calcium homeostasis in fast-twitch muscle. -

Animal Sarcoplasmic/Endoplasmic Reticulum-Type Calcium Pumps on the Primary Ca*+-Atpases of Red Beet
Plant Physiol. (1994)104: 1295-1300 A Study of the Effect of lnhibitors of the Animal Sarcoplasmic/Endoplasmic Reticulum-Type Calcium Pumps on the Primary Ca*+-ATPases of Red Beet linda J.Thomson, J. 1. Hall, and lorraine E. Williams'* Department of Biology, Biomedical Sciences Building, University of Southampton, Downloaded from https://academic.oup.com/plphys/article/104/4/1295/6067429 by guest on 29 September 2021 Southampton, SO9 3TU, United Kingdom 10 pmol mg-', it has little effect on the,hydrolysis of ATP by The inhibitor sensitivity of the endoplasmic reticulum (ER) and the Na+,K+-ATPase, the H+,K+-ATPase, the mitochondrial plasma membrane (PM) calcium pumps of red beet (Beta vulgaris FI-ATPase, and the erythrocyte Ca2+-ATPase(Seidler et al., 1.) were studied by measuring the ATP-driven accumulation of 1989). CPA is believed to inhibit the conformational transi- "CaZ+ into isolated membrane vesicles. Both transporters were tion of the enzyme between the E1 and E2 states (Seidler et strongly inhibited by 50 pmol m-3 erythrosin 6, but only by 50% in al., 1989). It prevents the binding of Ca2+to the high-affinity the presence of 100 mmol m-3 vanadate. A number of inhibitors site of the SR calcium pump and also inhibits Ca2+-dependent considered to be specific for the sarcoplasmic reticulum (SR)/ER- formation of phosphoenzyme (Goeger and Riley, 1989). The type calcium pump in animal cells were used to further characterize the PM and ER CaZ+-ATPasesin red beet and were compared with hydrophobic reagents nonylphenol, BHQ, AHQ, thapsigar- their effect on the transport and hydrolytic activities of the PM and gin, and trilobolide are also potent inhibitors of the SR tonoplast H+-ATPases. -

Supplementary Information For
Supplementary Information for Mechanisms for achieving high speed and efficiency in biomolecular machines Jason A. Wagoner and Ken A. Dill1 1To whom correspondence should be addressed. E-mail: dilllaufercenter.org This PDF file includes: Supplementary text Fig. S1 Tables S1 to S4 References for SI reference citations Jason A. Wagoner and Ken A. Dill1 1 of 13 www.pnas.org/cgi/doi/10.1073/pnas.1812149116 Supporting Information Text 1. Flux for the two-state model The steady-state flux (number of full cycles per unit time) can be derived from J = PBfm − PArm, where the transition rates are labelled in Figure 1 of the main text, and PA, PB are steady state probabilities that can be solved using the 2 × 2 rate matrix. Here, we give a derivation of the steady state flux that can be used for a more general network of states and for other kinetic properties, like higher moments of flux. The evolution of probability density along the periodic two-state model is ∂P (A , t) j = P (B , t) f + P (B , t) r ∂t j−1 m j c −P (Aj , t) (1 − fc − rm) , [S1] where P (Aj , t) is the probability density of state Aj at time t. The steady state flux can be calculated from the generating functions X −ijk P˜A (k, t) = e P (Aj , t) , [S2] j and similarly for P˜B (k, t). The time evolution of the generating function is ∂P˜A (k, t) X = M P˜ (k, t) , [S3] ∂t An n n∈{A,B} where −ik 1 − fc − rm rc + fme M = ik [S4] fc + rme 1 − fm − rc, and we are using lettered rather than numbered indices (MAB is M12, etc.). -
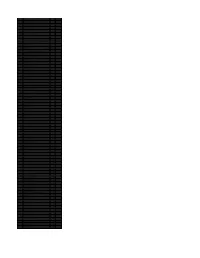
Accession Number Description Fold Enrichment Uniquepeptidecount
Accession Number Description Fold Enrichment UniquePeptideCount Q05BV5 OGFR protein 6.71 18 P24928 DNA-directed RNA polymerase II subunit RPB1 5.44 14 P07996 Thrombospondin-1 4.17 10 P30876 DNA-directed RNA polymerase II subunit RPB2 4.17 10 Q8WYQ5 Microprocessor complex subunit DGCR8 3.86 9 Q6IB91 PCK2 protein 3.54 8 Q9UN79 Transcription factor SOX-13 3.54 8 P18887 DNA repair protein XRCC1 3.22 7 P28331 NADH-ubiquinone oxidoreductase 75 kDa subunit mitochondrial 2.90 6 P49916 DNA ligase 3 2.90 6 P38646 Stress-70 protein mitochondrial 2.75 11 P13667 Protein disulfide-isomerase A4 2.59 5 Q9Y3Z3 SAM domain and HD domain-containing protein 1 2.59 5 Q12931 Heat shock protein 75 kDa mitochondrial 2.31 9 O00203 AP-3 complex subunit beta-1 2.27 4 O60231 Putative pre-mRNA-splicing factor ATP-dependent RNA helicase DHX16 2.27 4 P02751 Fibronectin 2.27 4 P09960 Leukotriene A-4 hydrolase 2.27 4 P10515 Dihydrolipoyllysine-residue acetyltransferase component of pyruvate dehydrogenase complex 2.27 4 P40763 Signal transducer and activator of transcription 3 2.27 4 P46459 Vesicle-fusing ATPase 2.27 4 P50542 Peroxisomal targeting signal 1 receptor 2.27 4 Q01082 Spectrin beta chain brain 1 2.27 4 Q15046 Lysine--tRNA ligase 2.27 4 Q2L6I2 ABC50 protein 2.27 4 Q52LR7 Enhancer of polycomb homolog 2 2.27 4 Q96L91 E1A-binding protein p400 2.27 4 Q9BYT8 Neurolysin mitochondrial 2.27 4 P43246 DNA mismatch repair protein Msh2 2.24 9 P06737 Glycogen phosphorylase liver form 2.17 8 P12956 X-ray repair cross-complementing protein 6 2.05 6 P40939 Trifunctional enzyme