MANAGEMENT RECOMMENDATIONS for WASHINGTON’S PRIORITY SPECIES – VOLUME V: MAMMALS (Interim)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Corynorhinus Townsendii): a Technical Conservation Assessment
Townsend’s Big-eared Bat (Corynorhinus townsendii): A Technical Conservation Assessment Prepared for the USDA Forest Service, Rocky Mountain Region, Species Conservation Project October 25, 2006 Jeffery C. Gruver1 and Douglas A. Keinath2 with life cycle model by Dave McDonald3 and Takeshi Ise3 1Department of Biological Sciences, University of Calgary, Calgary, Alberta, Canada 2Wyoming Natural Diversity Database, Old Biochemistry Bldg, University of Wyoming, Laramie, WY 82070 3Department of Zoology and Physiology, University of Wyoming, P.O. Box 3166, Laramie, WY 82071 Peer Review Administered by Society for Conservation Biology Gruver, J.C. and D.A. Keinath (2006, October 25). Townsend’s Big-eared Bat (Corynorhinus townsendii): a technical conservation assessment. [Online]. USDA Forest Service, Rocky Mountain Region. Available: http:// www.fs.fed.us/r2/projects/scp/assessments/townsendsbigearedbat.pdf [date of access]. ACKNOWLEDGMENTS The authors would like to acknowledge the modeling expertise of Dr. Dave McDonald and Takeshi Ise, who constructed the life-cycle analysis. Additional thanks are extended to the staff of the Wyoming Natural Diversity Database for technical assistance with GIS and general support. Finally, we extend sincere thanks to Gary Patton for his editorial guidance and patience. AUTHORS’ BIOGRAPHIES Jeff Gruver, formerly with the Wyoming Natural Diversity Database, is currently a Ph.D. candidate in the Biological Sciences program at the University of Calgary where he is investigating the physiological ecology of bats in northern arid climates. He has been involved in bat research for over 8 years in the Pacific Northwest, the Rocky Mountains, and the Badlands of southern Alberta. He earned a B.S. in Economics (1993) from Penn State University and an M.S. -

Id & Ecology of OC Bats by Stephanie Remington V2
Bats Found in Orange County by Stephanie Remington FOOD HABITAT ROOST* MIGRATION / HIBERNATION STATUS NOTES Family Phyllostomidae nose ornamentation (leaf); migratory (do not hibernate) (Leaf-nosed bats) Mexican long-tongued near night-blooming cactae & caves, mines, bldgs fall migration to maternity roosts in has moved north to S. Cal. as habitat is lost in Mexico and as we plant more exotic batΨ Choeronycteris Nectar, pollen O agavae colonial (up to ~50) Mexico & Central America cactae & agavae; fall & winter records in S. Cal.; sensitive to disturbance mexicana Family Molossidae visible tail; migratory (usually do not hibernate); colonial; females form maternity colonies during the spring and summer months (Free-tailed bats) Mexican free-tailed bat variety of crevices; colonial (100s - migratory in parts of its range; appears one of the most common bats in OC; year-round activity, although reduced in winter; high altitudes C Tadarida brasiliensis agricultural pests 20 million) to overwinter in OC adapts well to urban environments; one pup born in Jun-July Pocketed free-tailed batΨ primarily large variable, from desert scrub to crevices of rugged cliffs; considered resident in San Diego very similar to the Mexican free-tailed bat, but slightly larger and with ears connected at Nyctinomops O moths pine-oak forest colonial County; status unknown in OC the midline; single pup, born in late Jun-Jul femorosaccus Big free-tailed batΨ almost entirely large usu crevices in cliffs; known only from a couple of records in OC; intermediate in size between pocketed free- rugged, rocky habitats seasonal migrant O Nyctinomops macrotis moths colonial tail and western mastiff bats; one pup, born late spring Western mastiff batΨ cliffs, occ. -

Bat Rabies and Other Lyssavirus Infections
Prepared by the USGS National Wildlife Health Center Bat Rabies and Other Lyssavirus Infections Circular 1329 U.S. Department of the Interior U.S. Geological Survey Front cover photo (D.G. Constantine) A Townsend’s big-eared bat. Bat Rabies and Other Lyssavirus Infections By Denny G. Constantine Edited by David S. Blehert Circular 1329 U.S. Department of the Interior U.S. Geological Survey U.S. Department of the Interior KEN SALAZAR, Secretary U.S. Geological Survey Suzette M. Kimball, Acting Director U.S. Geological Survey, Reston, Virginia: 2009 For more information on the USGS—the Federal source for science about the Earth, its natural and living resources, natural hazards, and the environment, visit http://www.usgs.gov or call 1–888–ASK–USGS For an overview of USGS information products, including maps, imagery, and publications, visit http://www.usgs.gov/pubprod To order this and other USGS information products, visit http://store.usgs.gov Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government. Although this report is in the public domain, permission must be secured from the individual copyright owners to reproduce any copyrighted materials contained within this report. Suggested citation: Constantine, D.G., 2009, Bat rabies and other lyssavirus infections: Reston, Va., U.S. Geological Survey Circular 1329, 68 p. Library of Congress Cataloging-in-Publication Data Constantine, Denny G., 1925– Bat rabies and other lyssavirus infections / by Denny G. Constantine. p. cm. - - (Geological circular ; 1329) ISBN 978–1–4113–2259–2 1. -

Life History Account for Pallid
California Wildlife Habitat Relationships System California Department of Fish and Wildlife California Interagency Wildlife Task Group PALLID BAT Antrozous pallidus Family: VESPERTILIONIDAE Order: CHIROPTERA Class: MAMMALIA M038 Written by: J. Harris Reviewed by: P. Brown Edited by: D. Alley, R. Duke DISTRIBUTION, ABUNDANCE, AND SEASONALlTY The pallid bat is a locally common species of low elevations in California. It occurs throughout California except for the high Sierra Nevada from Shasta to Kern cos., and the northwestern corner of the state from Del Norte and western Siskiyou cos. to northern Mendocino Co. A wide variety of habitats is occupied, including grasslands, shrublands, woodlands, and forests from sea level up through mixed conifer forests. The species is most common in open, dry habitats with rocky areas for roosting. A yearlong resident in most of the range. SPECIFIC HABITAT REQUIREMENTS Feeding: Takes a wide variety of insects and arachnids, including beetles, orthopterans, homopterans, moths, spiders, scorpions, solpugids, and Jerusalem crickets. The stout skull and dentition of this species allows it to take large, hard-shelled prey. Forages over open ground, usually 0.5-2.5 m (1.6-8 ft) above ground level. Foraging flight is slow and maneuverable with frequent dips, swoops, and short glides. Many prey are taken on the ground. Gleaning is frequently used, and a few prey are taken aerially. Can maneuver well on the ground. May carry large prey to a perch or night roost for consumption. Ingestion of fruit in one study (Howell 1980) was a result of feeding on frugivorous moths. Uses echolocation for obstacle avoidance; possibly utilizes prey-produced sounds while foraging. -

Bciissue22018.Pdf
BAT CONSERVATION INTERNATIONAL ISSUE 2 • 2018 // BATCON.ORG CHIROPTERAN Research and development seeks to unlock and harness the secrets of bats’ techextraordinary capabilities THE CAVERN SPECIES SPOTLIGHT: THE SWEETEST OF YOUTH TRI-COLORED BAT FRUITS BECOME a MONTHLY SUSTAINING MEMBER Photo: Vivian Jones Vivian Photo: Grey-headed flying fox (Pteropus poliocephalus) When you choose to provide an automatic monthly donation, you allow BCI to plan our conservation programs with confidence, knowing the resources you and other sustaining members provide are there when we need them most. Being a Sustaining Member is also convenient for you, as your monthly gift is automatically transferred from your debit or credit card. It’s safe and secure, and you can change or cancel your allocation at any time. As an additional benefit, you won’t receive membership renewal requests, which helps us reduce our paper and postage costs. BCI Sustaining Members receive our Bats magazine, updates on our bat conservation efforts and an opportunity to visit Bracken Cave with up to five guests every year. Your consistent support throughout the year helps strengthen our organizational impact. TO BECOME A SUSTAINING MEMBER TODAY, VISIT BATCON.ORG/SUSTAINING OR SELECT SUSTAINING MEMBER ON THE DONATION ENVELOPE ENCLOSED WITH YOUR DESIRED MONTHLY GIFT AMOUNT. 02 }bats Issue 23 2017 20172018 ISSUE 2 • 2018 bats INSIDE THIS ISSUE FEATURES 08 CHIROPTERAN TECH For sky, sea and land, bats are inspiring waves of new technology THE CAVERN OF YOUTH 12 Bats could help unlock -

Chiropterology Division BC Arizona Trial Event 1 1. DESCRIPTION: Participants Will Be Assessed on Their Knowledge of Bats, With
Chiropterology Division BC Arizona Trial Event 1. DESCRIPTION: Participants will be assessed on their knowledge of bats, with an emphasis on North American Bats, South American Microbats, and African MegaBats. A TEAM OF UP TO: 2 APPROXIMATE TIME: 50 minutes 2. EVENT PARAMETERS: a. Each team may bring one 2” or smaller three-ring binder, as measured by the interior diameter of the rings, containing information in any form and from any source. Sheet protectors, lamination, tabs and labels are permitted in the binder. b. If the event features a rotation through a series of stations where the participants interact with samples, specimens or displays; no material may be removed from the binder throughout the event. c. In addition to the binder, each team may bring one unmodified and unannotated copy of either the National Bat List or an Official State Bat list which does not have to be secured in the binder. 3. THE COMPETITION: a. The competition may be run as timed stations and/or as timed slides/PowerPoint presentation. b. Specimens/Pictures will be lettered or numbered at each station. The event may include preserved specimens, skeletal material, and slides or pictures of specimens. c. Each team will be given an answer sheet on which they will record answers to each question. d. No more than 50% of the competition will require giving common or scientific names. e. Participants should be able to do a basic identification to the level indicated on the Official List. States may have a modified or regional list. See your state website. -

Wei:Layout 1.Qxd
Acta Chiropterologica, 10(1): 51–59, 2008 PL ISSN 1508-1109 © Museum and Institute of Zoology PAS doi: 10.3161/150811008X331081 Wing morphology, echolocation calls, diet and emergence time of black-bearded tomb bats (Taphozous melanopogon, Emballonuridae) from southwest China LI WEI1, NAIJIAN HAN2, LIBIAO ZHANG3, KRISTOFER M. HELGEN4, 5, STUART PARSONS6, SHANYI ZHOU7, and SHUYI ZHANG1, 8 1School of Life Science, East China Normal University, 3663 Zhongshan Beilu, Putuo, Shanghai 200062, China 2Institute of Zoology, Chinese Academy of Sciences, Datun Lu, Chaoyang, Beijing 100080, China 3Guangdong Entomological Institute, 105 Xingang Xilu, Haizhu, Guangzhou 510260, China 4Division of Mammals, National Museum of Natural History, NHB 390 MRC 108, Smithsonian Institution, P.O. Box 37012, Washington D.C. 20013–7012, USA 5Division of Environmental and Life Sciences, Macquarie University, Sydney, NSW 2109, Australia 6School of Biological Sciences, University of Auckland, Private Bag 92019, Auckland, New Zealand 7College of Life Science, Guangxi Normal University, 15 Yucai Lu, Qixing, Guilin 541004, China 8Corresponding author: E-mail: [email protected] We studied the wing morphology, echolocation calls, diet and emergence time of the black-bearded tomb bat (Taphozous melanopogon) from May to October 2006 in Guangxi Province, southwest China. Taphozous melanopogon has wings with high aspect ratio, high loading and pointed wing-tip shape-characteristics associated with fast flight in open space. This species usually produces low-intensity, low frequency, and frequency-modulated (FM) calls usually containing up to four harmonics, with most energy in the second (or sometimes third) harmonic. The diet of this species consists mostly of Lepidoptera and Hemiptera. -

Pallid Bat (Antrozous Pallidus) in Canada
PROPOSED Species at Risk Act Recovery Strategy Series Adopted under Section 44 of SARA Recovery Strategy for the Pallid Bat (Antrozous pallidus) in Canada Pallid Bat 2017 1 Recommended citation: Environment and Climate Change Canada. 2017. Recovery Strategy for the Pallid Bat (Antrozous pallidus) in Canada [Proposed]. Species at Risk Act Recovery Strategy Series. Environment and Climate Change Canada, Ottawa. 2 parts, 14 pp. + 46 pp. For copies of the recovery strategy, or for additional information on species at risk, including the Committee on the Status of Endangered Wildlife in Canada (COSEWIC) Status Reports, residence descriptions, action plans, and other related recovery documents, please visit the Species at Risk (SAR) Public Registry1. Cover illustration: © Barry Mansell Également disponible en français sous le titre « Programme de rétablissement de la chauve-souris blonde (Antrozous pallidus) au Canada [Proposition] » © Her Majesty the Queen in Right of Canada, represented by the Minister of Environment and Climate Change, 2017. All rights reserved. ISBN Catalogue no. Content (excluding the illustrations) may be used without permission, with appropriate credit to the source. 1 http://sararegistry.gc.ca/default.asp?lang=En&n=24F7211B-1 RECOVERY STRATEGY FOR THE PALLID BAT (Antrozous pallidus) IN CANADA 2017 Under the Accord for the Protection of Species at Risk (1996), the federal, provincial, and territorial governments agreed to work together on legislation, programs, and policies to protect wildlife species at risk throughout Canada. In the spirit of cooperation of the Accord, the Government of British Columbia has given permission to the Government of Canada to adopt the Recovery Plan for the Pallid Bat (Antrozous pallidus) in British Columbia (Part 2) under Section 44 of the Species at Risk Act (SARA). -

The Post-Release Fate of Hand-Reared Orphaned Bats: Survival and Habitat Selection MT Serangeli†, L Cistrone‡, L Ancillotto§, a Tomassini§ and D Russo*†
9 © 2012 Universities Federation for Animal Welfare Animal Welfare 2012, 21: 9-18 The Old School, Brewhouse Hill, Wheathampstead, ISSN 0962-7286 Hertfordshire AL4 8AN, UK The post-release fate of hand-reared orphaned bats: survival and habitat selection MT Serangeli†, L Cistrone‡, L Ancillotto§, A Tomassini§ and D Russo*†# † Laboratorio di Ecologia Applicata, Dipartimento Ar.Bo.Pa.Ve, Facoltà di Agraria, Università degli Studi di Napoli Federico II, via Università 100, I-80055, Portici, Naples, Italy ‡ Forestry and Conservation, Via Botticelli 14, I-03043 Cassino, Frosinone, Italy § Dipartimento di Biologia e Biotecnologia ‘Charles Darwin’, Università degli Studi di Roma ‘La Sapienza’, Viale Università 32, I-00185 Rome, Italy # School of Biological Sciences, University of Bristol, Woodland Road, Bristol BS8 1UG, UK * Contact for correspondence and requests for reprints: [email protected] Abstract Although bats are frequently admitted to rescue centres — mainly as orphans — very little information is available on their survival after release. Our study answered the following questions: i) do hand-reared bats survive over a short time; ii) which activities and habitat selection do they exhibit; iii) are bats loyal to the release area; and iv) are they able to join local colonies? We radio-tracked 21 hand-reared Pipistrellus kuhlii over a two-year period released on a site that differed from that where they were rescued. At the study site they were provided with the same bat boxes used in the rehabilitation room. Nineteen bats were confirmed to survive, stay in the area and actively forage over 4–14 days. Fourteen day roosts in buildings (nine of which hosted a local colony) were used by 12 subjects. -

Pallid Bat Detection of Dangerous Prey
A Sting in the Night: Pallid Bat Detection of Dangerous Prey Nicholas Carlson: McNair Scholar Dr. Jesse Barber: Mentor Biology Abstract It has been observed in previous studies that Hemprich long-eared bats (Otonycteris hemprichii) are frequently stung during predation attacks on scorpions. Although a highly toxic and dangerous prey, the scorpion toxicity does not kill the bat. The sting, however, does seem to inflict a great amount of pain. Here, we examine the role of bat vision in predator-prey interactions between a similar bat species, pallid bats (Antrozous pallidus) and northern scorpions (Paruroctonus boreus). We address the question: do bats use visual information provided by moonlight to plan attacks on dangerous prey? We predicted that under moonlit conditions, pallid bats would plan attacks on scorpion prey, therefore, being stung less often. Our experiments took place in a flight room in both simulated moonlight and complete darkness. The interactions were recorded live by three high-definition cameras mounted in three separate corners of the interaction arena. Keywords: Sensory Ecology, Pallid Bats, Bruneau Sand Dunes, Northern Scorpions, Passive Listening Sensory Ecology Sensory ecology is a relatively new field of ecology that focuses on how animals acquire, process, and use sensory information, and the sensory systems involved. Tasks such as finding food, avoiding predators, attracting mates, and navigating through complex environments are all governed by sensory systems. Subsequently, animals have evolved an astounding range of sensory organs that are crucial to survival and reproduction. These sensory organs determine much of their evolution and behavior. Sensory ecologists investigate the type of sensory information that is gathered by animals, how it is used in a range of behaviors, and the evolution of these traits. -
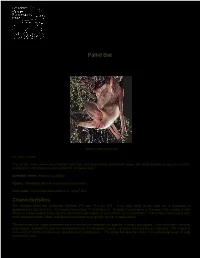
Northwestwildlife.Com Species Reports
A publication by: NORTHWEST WILDLIFE PRESERVATION SOCIETY Pallid Bat Antrozous pallidus Photo credit: Earthlink By Julie Whittet One of the rarest mammals of British Columbia, this large-eared species of vesper bat hunts its prey using echolocation and gets its name from the ivory colour of its stomach fur. Scientific name: Antrozous pallidus Family: Vespertilionidae (evening and vesper bats) Nick name: Sometimes described as a “ghost” bat Characteristics The average pallid bat measures between 9.2 and 13.5 cm (3.5 - 5 in) from head to tail, and has a wingspan of approximately 35 cm (13in). Its weight ranges from 17 to 28 grams. Its body is covered by a fine woolly fur, usually a light brown or cream-yellow colour on the back and a pale white or ivory colour on its underside. They have a blunt snout with scroll shaped nostrils. Male and female individuals are typically similar in appearance. The pallid bat has highly prominent ears, a specialized adaption for superior auditory perception. Their ears have serrated outer edges, pointed tips and are distinguished by an elongated tragus – a tissue covering the ear opening. The tragus is believed to help the bat perceive sound through echolocation. The pallid bat also has large eyes and sharp vision to help them locate prey. www.northwestwildlife.com t: 604.568.9160 f: 604.568.6152 [email protected] The pallid bat can be distinguished from other bats by its larger body, ears and eyes as well as its pale colouring. Unlike big-eared bats, the ears of the pallid bat are not joined at the base. -

List of 28 Orders, 129 Families, 598 Genera and 1121 Species in Mammal Images Library 31 December 2013
What the American Society of Mammalogists has in the images library LIST OF 28 ORDERS, 129 FAMILIES, 598 GENERA AND 1121 SPECIES IN MAMMAL IMAGES LIBRARY 31 DECEMBER 2013 AFROSORICIDA (5 genera, 5 species) – golden moles and tenrecs CHRYSOCHLORIDAE - golden moles Chrysospalax villosus - Rough-haired Golden Mole TENRECIDAE - tenrecs 1. Echinops telfairi - Lesser Hedgehog Tenrec 2. Hemicentetes semispinosus – Lowland Streaked Tenrec 3. Microgale dobsoni - Dobson’s Shrew Tenrec 4. Tenrec ecaudatus – Tailless Tenrec ARTIODACTYLA (83 genera, 142 species) – paraxonic (mostly even-toed) ungulates ANTILOCAPRIDAE - pronghorns Antilocapra americana - Pronghorn BOVIDAE (46 genera) - cattle, sheep, goats, and antelopes 1. Addax nasomaculatus - Addax 2. Aepyceros melampus - Impala 3. Alcelaphus buselaphus - Hartebeest 4. Alcelaphus caama – Red Hartebeest 5. Ammotragus lervia - Barbary Sheep 6. Antidorcas marsupialis - Springbok 7. Antilope cervicapra – Blackbuck 8. Beatragus hunter – Hunter’s Hartebeest 9. Bison bison - American Bison 10. Bison bonasus - European Bison 11. Bos frontalis - Gaur 12. Bos javanicus - Banteng 13. Bos taurus -Auroch 14. Boselaphus tragocamelus - Nilgai 15. Bubalus bubalis - Water Buffalo 16. Bubalus depressicornis - Anoa 17. Bubalus quarlesi - Mountain Anoa 18. Budorcas taxicolor - Takin 19. Capra caucasica - Tur 20. Capra falconeri - Markhor 21. Capra hircus - Goat 22. Capra nubiana – Nubian Ibex 23. Capra pyrenaica – Spanish Ibex 24. Capricornis crispus – Japanese Serow 25. Cephalophus jentinki - Jentink's Duiker 26. Cephalophus natalensis – Red Duiker 1 What the American Society of Mammalogists has in the images library 27. Cephalophus niger – Black Duiker 28. Cephalophus rufilatus – Red-flanked Duiker 29. Cephalophus silvicultor - Yellow-backed Duiker 30. Cephalophus zebra - Zebra Duiker 31. Connochaetes gnou - Black Wildebeest 32. Connochaetes taurinus - Blue Wildebeest 33. Damaliscus korrigum – Topi 34.