Final Updated Petroleum Hydrocarbon Fraction Toxicity Values for the Vph
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
![C9-14 Aliphatic [2-25% Aromatic] Hydrocarbon Solvents Category SIAP](https://docslib.b-cdn.net/cover/2852/c9-14-aliphatic-2-25-aromatic-hydrocarbon-solvents-category-siap-12852.webp)
C9-14 Aliphatic [2-25% Aromatic] Hydrocarbon Solvents Category SIAP
CoCAM 2, 17-19 April 2012 BIAC/ICCA SIDS INITIAL ASSESSMENT PROFILE Chemical C -C Aliphatic [2-25% aromatic] Hydrocarbon Solvents Category Category 9 14 Substance Name CAS Number Stoddard solvent 8052-41-3 Chemical Names Kerosine, petroleum, hydrodesulfurized 64742-81-0 and CAS Naphtha, petroleum, hydrodesulfurized heavy 64742-82-1 Registry Solvent naphtha, petroleum, medium aliphatic 64742-88-7 Numbers Note: Substances in this category are also commonly known as mineral spirits, white spirits, or Stoddard solvent. CAS Number Chemical Description † 8052-41-3 Includes C8 to C14 branched, linear, and cyclic paraffins and aromatics (6 to 18%), <50ppmV benzene † 64742-81-0 Includes C9 to C14 branched, linear, and cyclic paraffins and aromatics (10 to Structural 25%), <100 ppmV benzene Formula † and CAS 64742-82-1 Includes C8 to C13 branched, linear, and cyclic paraffins and aromatics (15 to 25%), <100 ppmV benzene Registry † Numbers 64742-88-7 Includes C8 to C13 branched, linear, and cyclic paraffins and aromatics (14 to 20%), <50 ppmV benzene Individual category member substances are comprised of aliphatic hydrocarbon molecules whose carbon numbers range between C9 and C14; approximately 80% of the aliphatic constituents for a given substance fall within the C9-C14 carbon range and <100 ppmV benzene. In some instances, the carbon range of a test substance is more precisely defined in the test protocol. In these instances, the specific carbon range (e.g. C8-C10, C9-C10, etc.) will be specified in the SIAP. * It should be noted that other substances defined by the same CAS RNs may have boiling ranges outside the range of 143-254° C and that these substances are not covered by the category. -

Products of OH + Furan Reactions and Some Implications for Aromatic Hydrocarbon Atmospheric Degradation
Products of OH + Furan Reactions and Some Implications for Aromatic Hydrocarbon Atmospheric Degradation Roger Atkinson, Air Pollution Research Center, University of California, Riverside Unsaturated 1,4-dicarbonyls are important products of the atmospheric degradations of aromatic hydrocarbons such as benzene, toluene, xylenes and trimethylbenzenes, which comprise 20% of the non- methane volatile organic compounds present in urban air masses in the USA. However, in many cases the measured formation yields of the unsaturated 1,4-dicarbonyls are significantly lower than those of these presumed co-product 1,2-dicarbonyls. These discrepancies could be due to analytical problems and/or rapid photolysis of unsaturated 1,4-keto-aldehydes and unsaturated 1,4-dialdehydes, or to incorrect reaction mechanisms for the OH radical-initiated reactions of aromatic hydrocarbons. Since unsaturated 1,4- dicarbonyls are major products of OH + furans, with apparently simpler product distributions and mechanisms, we have investigated the reactions of OH radicals with furan, 2- and 3-methylfuran, and 2,3- and 2,5- dimethylfuran, in the presence of NO. Using direct air sampling atmospheric pressure ionization tandem mass spectrometry and gas chromatography, the unsaturated 1,4-dicarbonyls were observed and quantified. The measured unsaturated 1,4-dicarbonyl formation yields ranged from 8 ± 2% from OH + 2,3-dimethylfuran to 75 ± 5% from OH + furan. Other products were also formed. These data will be presented and discussed and, time permitting, a brief discussion of in situ nitro-aromatic and nitro-PAH formation from the atmospheric degradations of aromatic hydrocarbons and PAHs will also be presented. Live cast on the link below: http://www.fin.ucar.edu/it/mms/fl-live.htm A recording will be available on ACD’s website for viewing at a later date. -

Formation of Highly Oxygenated Organic Molecules from Aromatic Compounds
Formation of highly oxygenated organic molecules from aromatic compounds. Ugo Molteni1, Federico Bianchi1-2, Felix Klein1, Imad El Haddad1, Carla Frege1, Michel J. Rossi1, Josef Dommen1, Urs Baltensperger1,* 5 1Laboratory of Atmospheric Chemistry, Paul Scherrer Institute, CH-5232 Villigen, Switzerland 2Department of Physics, University of Helsinki, 00014 Helsinki, Finland Correspondence to: Urs Baltensperger ([email protected]) Abstract 10 Anthropogenic volatile organic compounds (AVOC) often dominate the urban atmosphere and consist to a large degree of aromatic hydrocarbons (ArHC), such as benzene, toluene, xylenes, and trimethylbenzenes, e.g. from handling and combustion of fuels. These compounds are important precursors for the formation of secondary organic aerosol. Despite their recognized importance as atmospheric reactants, the formation of highly oxygenated molecules (HOMs) in the gas phase leading to (extremely) low volatility compounds has not been studied in the past. Here we show that oxidation of 15 aromatics with OH leads to a subsequent autoxidation chain reaction forming HOMs with an O:C ratio of up to 1.09. This is exemplified for five single-ring ArHC (benzene, toluene, o-/m-/p-xylene, mesitylene (1,3,5-trimethylbenzene) and ethylbenzene), as well as two conjugated polycyclic ArHC (naphthalene and biphenyl). We present the identified compounds, differences in the observed oxidation patterns and discuss mechanistic pathways. We report the elemental composition of the HOMs and show the differences in the oxidation patterns of these ArHCs. A potential pathway for the 20 formation of these HOMs from aromatics is presented and discussed. We hypothesize that AVOC may contribute substantially to new particle formation events that have been detected in urban areas. -

Stoddard Solvent
DRAFT TOXICOLOGICAL PROFILE FOR STODDARD SOLVENT Prepared by: Clement International Corporation Under Contract No. 205-88-0608 Prepared for: U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service Agency for Toxic Substances and Disease Registry May 1993 ***DRAFT FOR PUBLIC COMMENT*** DISCLAIMER The use of company or product name(s) is for identification only and does not imply endorsement by the Agency for Toxic Substances and Disease Registry. ***DRAFT FOR PUBLIC COMMENT*** iii FOREWORD The Superfund Amendments and Reauthorization Act (SARA) of 1986 (Public Law 99-499) amended the Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA or Superfund). Section 211 of SARA also amended Title 10 of the U.S. Code, creating the Defense Environmental Restoration Program. Section 2704(a) of Title 10 of the U.S. Code directs the Secretary of Defense to notify the Secretary of Health and Human Services of not less than 25 of the most commonly found unregulated hazardous substances at defense facilities. Section 2704(b) of Title 10 of the U.S. Code directs the Administrator of the Agency for Toxic Substances and Disease Registry (ATSDR) to prepare a toxicological proflle for each substance on the list provided by the Secretary of Defense under subsection (b). Each profile must include the following: (A) The examination, summary, and interpretation of available toxicological information and epidemiological evaluations on a hazardous substance in order to ascertain the levels of significant human exposure for the substance and the associated acute, subacute, and chronic health effects. (B) A determination of whether adequate information on the health effects of each substance is available or in the process of development to determine levels of exposure which present a significant risk to human health of acute, subacute, and chronic health effects. -

Trimethylbenzenes CAS Registry Numbers: 526-73-6 (1,2,3-TMB) 95-63-6 (1,2,4-TMB) 108-67-8 (1,3,5-TMB) 25551-13-7 (Mixed Isomers)
Development Support Document Final, September 4, 2015 Trimethylbenzenes CAS Registry Numbers: 526-73-6 (1,2,3-TMB) 95-63-6 (1,2,4-TMB) 108-67-8 (1,3,5-TMB) 25551-13-7 (Mixed Isomers) Prepared by Joseph T. Haney, Jr., M.S. Angela Curry, M.S. Toxicology Division Office of the Executive Director TEXAS COMMISSION ON ENVIRONMENTAL QUALITY Trimethylbenzenes Page i TABLE OF CONTENTS TABLE OF CONTENTS ............................................................................................................................................ I LIST OF TABLES ......................................................................................................................................................II ACRONYMS AND ABBREVIATIONS ................................................................................................................. III CHAPTER 1 SUMMARY TABLES .......................................................................................................................... 1 CHAPTER 2 MAJOR SOURCES AND USES ......................................................................................................... 4 CHAPTER 3 ACUTE EVALUATION ...................................................................................................................... 4 ACUTE 3.1 HEALTH-BASED ACUTE REV AND ESL ........................................................................................................ 4 3.1.1 Physical/Chemical Properties .................................................................................................................... -

Phase Equilibria of Supercritical Carbon Dioxide and Hydrocarbon Mixtures
Louisiana State University LSU Digital Commons LSU Historical Dissertations and Theses Graduate School 1992 Phase Equilibria of Supercritical Carbon Dioxide and Hydrocarbon Mixtures. Hyo-guk Lee Louisiana State University and Agricultural & Mechanical College Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_disstheses Recommended Citation Lee, Hyo-guk, "Phase Equilibria of Supercritical Carbon Dioxide and Hydrocarbon Mixtures." (1992). LSU Historical Dissertations and Theses. 5325. https://digitalcommons.lsu.edu/gradschool_disstheses/5325 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Historical Dissertations and Theses by an authorized administrator of LSU Digital Commons. For more information, please contact [email protected]. INFORMATION TO USERS This manuscript has been reproduced from the microfilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter face, while others may be from any type of computer printer. The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely afreet reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. Oversize materials (e.g., maps, drawings, charts) are reproduced by sectioning the original, beginning at the upper left-hand corner and continuing from left to right in equal sections with small overlaps. -

Environmental Health Criteria 122 N-Hexane
Environmental Health Criteria 122 n-Hexane Please note that the layout and pagination of this web version are not identical with the printed version. Hexane, n- (EHC 122, 1991) INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY ENVIRONMENTAL HEALTH CRITERIA 122 n-HEXANE This report contains the collective views of an international group of experts and does not necessarily represent the decisions or the stated policy of the United Nations Environment Programme, the International Labour Organisation, or the World Health Organization. Published under the joint sponsorship of the United Nations Environment Programme, the International Labour Organisation, and the World Health Organization First draft prepared by Dr. K. Chipman, University of Birmingham, United Kingdom World Health Orgnization Geneva, 1991 The International Programme on Chemical Safety (IPCS) is a joint venture of the United Nations Environment Programme, the International Labour Organisation, and the World Health Organization. The main objective of the IPCS is to carry out and disseminate evaluations of the effects of chemicals on human health and the quality of the environment. Supporting activities include the development of epidemiological, experimental laboratory, and risk-assessment methods that could produce internationally comparable results, and the development of manpower in the field of toxicology. Other activities carried out by the IPCS include the development of know-how for coping with chemical accidents, coordination of laboratory testing and epidemiological studies, and promotion of research on the mechanisms of the biological action of chemicals. WHO Library Cataloguing in Publication Data n-Hexane. (Environmental health criteria ; 122) Page 1 of 107 Hexane, n- (EHC 122, 1991) 1.Hexanes - adverse effects 2.Hexanes - toxicity I.Series ISBN 92 4 157122 5 (NLM Classification: QV 633) ISSN 0250-863X The World Health Organization welcomes requests for permission to reproduce or translate its publications, in part or in full. -

BUBBLE FORMATION in SUPERSATURATED HYDROCARBON MIXTURES Petroleum Reservoirs, the Mineral and Water Surfaces with for Each Crystal Averaging About 4.5 Sq Cm
T.P. 3441 BUBBLE FORMATION IN SUPERSATURATED HYDRO CARBON MIXTURES HARVEY T. KENNEDY, A AND M COlLEGE OF TEXAS, COlLEGE STATION, TEX., MEMBER AIME, AND CHARLES R. OLSON, OHIO OIL CO., SHREVEPORT, LA., JUNIOR MEMBER AIME Downloaded from http://onepetro.org/jpt/article-pdf/4/11/271/2238408/spe-232-g.pdf by guest on 29 September 2021 ABSTRACT tude, may be calculated for any rate of pressure decline imposed on the reservoir by production. The bearing of the In many investigations of the performance of petroleum res number and distribution of bubbles on reservoir performance ervoirs the assumption is made that the liquid, if below its is discussed. bubble-point pressure, is at all times in equilibrium with gas_ On the other hand, observations by numerous investigators have indicated that gas-liquid systems including hydrocarbon systems, may exhibit supersaturation to the extent of many INTRODUCTION hundred psi in the laboratory. Up to the present, there has been no reliable data on which to judge the actual extent of A liquid system is supersaturated with gas when the amount supersaturation under conditions approaching those existing of gas dissolved exceeds that corresponding to equilibrium at in petroleum reservoirs. the existing pressure and temperature. The degree of super The work reported here deals with observations and meas saturation may be conveniently expressed as the difference urements on mixtures of methane and kerosene in the presence between the bubble-point of the mixture and the prevailing of silica and calcite crystals. Bubbles were observed to form pressure. Thus, if a mixture having a bubble-point of 1,000 on crystal-hydrocarbon surfaces in preference to the glass psi at a given temperature exists in single liquid phase at hydrocarbon interface or to the body of the liquid. -

Stoddard Solvent 105 6. Analytical Methods 6.2
STODDARD SOLVENT 105 6. ANALYTICAL METHODS 6.2 ENVIRONMENTAL SAMPLES As with biological materials, detection of Stoddard solvent in environmental samples is based on the detection of component hydrocarbons. See Table 6-2 for a summary of the analytical methods used to determine Stoddard solvent hydrocarbons in environmental samples. The primary method for detecting volatile components of Stoddard solvent in air is GC using a flame ionization detector (FID) (NIOSH 1984; Otson et al. 1983). Stoddard solvent in air may be determined by absorption to an appropriate column such as charcoal, desorption in a solvent (carbon disulfide is recommended), and subsequent quantification. Although the precision of this method is good (greater than 10% relative standard deviation when the recovery is greater than 80%), in general, recovery tends to be rather poor (18-80%) because of the slow volatilization of Stoddard solvent (Otson et al. 1983). No analytical methods specific for Stoddard solvent in water or soil samples were located; however, determination of Stoddard solvent may be assumed to be similar to the detection of comparable hydrocarbon mixtures. Detection of Stoddard solvent in water is dependent on the identification and quantification of the specific hydrocarbon components of the solvent. The primary method, GC either alone or in combination with MS, may be used for the identification of the major hydrocarbon components, i.e., n-alkanes, branched alkanes, cycloalkanes, and alkylbenzenes. Separation of the aliphatic and aromatic fractions may be achieved by liquid-solid column chromatography followed by dilution of the eluates with carbon disulfide. Aqueous samples may be extracted with trichlorotrifluoroethane, while solid samples may be extracted by Soxhlet extraction or sonication methods (Air Force 1989). -

Impact of Molecular Structure on Secondary Organic Aerosol Formation from Aromatic Hydrocarbon
Impact of Molecular Structure on Secondary Organic Aerosol Formation from Aromatic Hydrocarbon Photooxidation under Low NOX Conditions L. Li1,2, P. Tang1,2, S. Nakao1,2,3 and D. R. Cocker III1,2 5 [1] University of California, Riverside, Department of Chemical and Environmental Engineering, Riverside, CA 92507, USA [2] College of Engineering-Center for Environmental Research and Technology (CE- CERT), Riverside, CA 92507, USA [3] Currently at Clarkson University, Department of Chemical and Biomolecular 10 Engineering, Potsdam, NY 13699, USA Correspondence to: D. Cocker III ([email protected]) Abstract The molecular structure of volatile organic compounds (VOC) determines their oxidation pathway, directly impacting secondary organic aerosol (SOA) formation. This study 15 comprehensively investigates the impact of molecular structure on SOA formation from the photooxidation of twelve different eight- to nine-carbon aromatic hydrocarbons under low NOx conditions. The effects of the alkyl substitute number, location, carbon chain length and branching structure on the photooxidation of aromatic hydrocarbons are demonstrated by analyzing SOA yield, chemical composition and physical properties. Aromatic hydrocarbons, 20 categorized into five groups, show a yield order of ortho (o-xylene and o-ethyltoluene)> one substitute (ethylbenzene, propylbenzene and isopropylbenzene) > meta (m-xylene and m- ethyltoluene)> three substitute (trimethylbenzenes) > para (p-xylene and p-ethyltoluene). SOA yields of aromatic hydrocarbon photooxidation do not monotonically decrease when increasing alkyl substitute number. The ortho position promotes SOA formation while the 25 para position suppresses aromatic oxidation and SOA formation. Observed SOA chemical composition and volatility confirm that higher yield is associated with further oxidation. SOA chemical composition also suggests that aromatic oxidation increases with increasing alkyl substitute chain length and branching structure. -

Industrial Hydrocarbon Processes
Handbook of INDUSTRIAL HYDROCARBON PROCESSES JAMES G. SPEIGHT PhD, DSc AMSTERDAM • BOSTON • HEIDELBERG • LONDON NEW YORK • OXFORD • PARIS • SAN DIEGO SAN FRANCISCO • SINGAPORE • SYDNEY • TOKYO Gulf Professional Publishing is an imprint of Elsevier Gulf Professional Publishing is an imprint of Elsevier The Boulevard, Langford Lane, Kidlington, Oxford OX5 1GB, UK 30 Corporate Drive, Suite 400, Burlington, MA 01803, USA First edition 2011 Copyright Ó 2011 Elsevier Inc. All rights reserved No part of this publication may be reproduced, stored in a retrieval system or transmitted in any form or by any means electronic, mechanical, photocopying, recording or otherwise without the prior written permission of the publisher Permissions may be sought directly from Elsevier’s Science & Technology Rights Department in Oxford, UK: phone (+44) (0) 1865 843830; fax (+44) (0) 1865 853333; email: [email protected]. Alternatively you can submit your request online by visiting the Elsevier web site at http://elsevier.com/locate/ permissions, and selecting Obtaining permission to use Elsevier material Notice No responsibility is assumed by the publisher for any injury and/or damage to persons or property as a matter of products liability, negligence or otherwise, or from any use or operation of any methods, products, instructions or ideas contained in the material herein. Because of rapid advances in the medical sciences, in particular, independent verification of diagnoses and drug dosages should be made British Library Cataloguing in Publication Data -
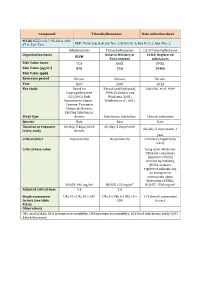
Compound Trimethylbenzenes Data Collection Sheet N°CAS
Compound Trimethylbenzenes Data collection sheet N°CAS 25551-13-7, 95-63-6, 108- 67-8, 526-73-8 CLP: Flam. Liq. 3, Acute Tox. 4, Skin Irrit. 2, Eye Irrit. 2, Asp. Tox. 1, Alkylbenzenes Trimethylbenzenes 1,2,4-Trimethylbenzene Organization Name Ontario Ministry of ECHA: Registered RIVM Environment substances Risk Value Name TCA AAQC DNEL Risk Value (µg/m3) 870 220 29400 Risk Value (ppb) Reference period Chronic Chronic Chronic Year 2007 2007 2013 Key Study Based on Korsak and Rydzynski, Clark DG, et al. 1989 Isopropylbenzene 1996; Gralewicz and EU (2001) Risk Wiaderna, 2001; Assessement Report – Wiaderna et al., 2002 Cumene. European Chemicals Bureau, Existing Substances. Study type chronic Subchronic inhalation Chronic inhalation Species Rats Rats Rats Duration of exposure 6h/day, 5 days/week 6h/day, 5 days/week 6h/day, 5 days/week, 1 in key study chronic year Critical effect Neurotoxicity Neurotoxicity Irritation (respiratory tract) Critical dose value Long term inhalation DNEL for consumers (systemic effects) derived by industry (ECHA-website: registered substances), no transparent information about derivation of DNEL NOAEL 490 mg/m3 NOAEL 123 mg/m³ NOAEC 1800 mg/m3 Adjusted critical dose 5.6 5.6 Single assessment UFH 10 x UFA 10 = 100 UFs 3 x UFH 3 x UFA 10 = 1.7 (Overall assessment factors (see table 100 factor) R.8.6) Other effects UFL used LOAEL; UFH intraspecies variability; UFA interspecies variability; UFS Used subchronic study; UFD data deficiencies Compound TRIMETHYLBENZENES Factsheet Parameter Note Comments Value / descriptor EU-LCI Value and Status EU-LCI value 1 Mass/volume [µg/m³] 450 EU-LCI status 2 Draft / Final Final Year when the EU-LCI value has been EU-LCI year of issue 3 2012 issued General Information 601-025-00-5 CLP-INDEX-Nr.