Alfred L. Rosenberger Fossil New World Monkeys Dispute the Molecular Clock
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Development Team
Paper No. : 14 Human Origin and Evolution Module : 09 Classification and Distribution of Living Primates Development Team Principal Investigator Prof. Anup Kumar Kapoor Department of Anthropology, University of Delhi Dr. Satwanti Kapoor (Retd Professor) Paper Coordinator Department of Anthropology, University of Delhi Mr. Vijit Deepani & Prof. A.K. Kapoor Content Writer Department of Anthropology, University of Delhi Prof. R.K. Pathak Content Reviewer Department of Anthropology, Panjab University, Chandigarh 1 Classification and Distribution of Living Primates Anthropology Description of Module Subject Name Anthropology Paper Name Human Origin and Evolution Module Name/Title Classification and Distribution of Living Primates Module Id 09 Contents: Primates: A brief Outline Classification of Living Primates Distribution of Living Primates Summary Learning Objectives: To understand the classification of living primates. To discern the distribution of living primates. 2 Classification and Distribution of Living Primates Anthropology Primates: A brief Outline Primates reside at the initial stage in the series of evolution of man and therefore constitute the first footstep of man’s origin. Primates are primarily mammals possessing several basic mammalian features such as presence of mammary glands, dense body hair; heterodonty, increased brain size, endothermy, a relatively long gestation period followed by live birth, considerable capacity for learning and behavioural flexibility. St. George J Mivart (1873) defined Primates (as an order) -

Early Miocene Paleobiology in Patagonia. High-Latitude Paleocommunities of the Santa Cruz Formation Sergio F
Early Miocene Paleobiology in Patagonia. High-Latitude Paleocommunities of the Santa Cruz Formation Sergio F. Vizcaino, Richard F. Kay, and M. Susana Bargo (eds.) Cambridge, UK: Cambridge University Press, 2012, 370 pp. (hardback), $155.00. ISBN-13: 9780521194617. Reviewed by SUSAN CACHEL Department of Anthropology, Rutgers University, New Brunswick, NJ 08901-1414, USA; [email protected] his edited volume deals with new fossil flora and fauna of these chapters. These appendices list museum catalog Tfrom the Atlantic Coast of Patagonia, South America, numbers, along with collection area and short descriptions. dating to 18–16 mya. Two of the editors are affiliated with This volume therefore is a crucial resource for researchers the Museo de la Plata, Argentina, and a plethora of Argen- studying mammal evolution, especially for those studying tine colleagues contribute chapters and reviewed prelimi- processes like convergent evolution. New black-and-white nary drafts. The volume thus has a spectacularly interna- illustrations reconstruct details of life on the ancient land- tional authorship. Abstracts of each chapter appear in both scapes. English and Spanish. The collection of the fossils is an epic in itself. Because In contrast to the cold and dismal climate of modern fossils were embedded in sandstone beach rock in the in- Patagonia, Patagonia 18–16 mya was much warmer and hu- tertidal zone, retrieval of these specimens often entailed mid, with mixed forests and grasslands. The Andes Moun- hastily using both geological hammers and jack hammers tains were much lower in elevation, which allowed an east- to remove blocks of sediment before the tide turned, and ward expansion of habitats now found today on the lower cold Atlantic seawater again swept over the collecting area. -

Genome Sequence of the Basal Haplorrhine Primate Tarsius Syrichta Reveals Unusual Insertions
ARTICLE Received 29 Oct 2015 | Accepted 17 Aug 2016 | Published 6 Oct 2016 DOI: 10.1038/ncomms12997 OPEN Genome sequence of the basal haplorrhine primate Tarsius syrichta reveals unusual insertions Ju¨rgen Schmitz1,2, Angela Noll1,2,3, Carsten A. Raabe1,4, Gennady Churakov1,5, Reinhard Voss6, Martin Kiefmann1, Timofey Rozhdestvensky1,7,Ju¨rgen Brosius1,4, Robert Baertsch8, Hiram Clawson8, Christian Roos3, Aleksey Zimin9, Patrick Minx10, Michael J. Montague10, Richard K. Wilson10 & Wesley C. Warren10 Tarsiers are phylogenetically located between the most basal strepsirrhines and the most derived anthropoid primates. While they share morphological features with both groups, they also possess uncommon primate characteristics, rendering their evolutionary history somewhat obscure. To investigate the molecular basis of such attributes, we present here a new genome assembly of the Philippine tarsier (Tarsius syrichta), and provide extended analyses of the genome and detailed history of transposable element insertion events. We describe the silencing of Alu monomers on the lineage leading to anthropoids, and recognize an unexpected abundance of long terminal repeat-derived and LINE1-mobilized transposed elements (Tarsius interspersed elements; TINEs). For the first time in mammals, we identify a complete mitochondrial genome insertion within the nuclear genome, then reveal tarsier-specific, positive gene selection and posit population size changes over time. The genomic resources and analyses presented here will aid efforts to more fully understand the ancient characteristics of primate genomes. 1 Institute of Experimental Pathology, University of Mu¨nster, 48149 Mu¨nster, Germany. 2 Mu¨nster Graduate School of Evolution, University of Mu¨nster, 48149 Mu¨nster, Germany. 3 Primate Genetics Laboratory, German Primate Center, Leibniz Institute for Primate Research, 37077 Go¨ttingen, Germany. -

Xenothrix Mcgregori (Xenotrichini, Callicebinae, Pitheciidae), with a Reconsideration of the Aotus Hypothesis 1
New Craniodental Remains of the Quaternary Jamaican Monkey Xenothrix mcgregori (Xenotrichini, Callicebinae, Pitheciidae), with a Reconsideration of the Aotus Hypothesis 1 Authors: MACPHEE, R.D.E., and HOROVITZ, INÉS Source: American Museum Novitates, 2004(3434) : 1-51 Published By: American Museum of Natural History URL: https://doi.org/10.1206/0003- 0082(2004)434<0001:NCROTQ>2.0.CO;2 BioOne Complete (complete.BioOne.org) is a full-text database of 200 subscribed and open-access titles in the biological, ecological, and environmental sciences published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Complete website, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/terms-of-use. Usage of BioOne Complete content is strictly limited to personal, educational, and non - commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. Downloaded From: https://bioone.org/journals/American-Museum-Novitates on 01 Apr 2020 Terms of Use: https://bioone.org/terms-of-use Access provided by American Museum of Natural History PUBLISHED BY THE AMERICAN MUSEUM OF NATURAL HISTORY CENTRAL PARK WEST AT 79TH STREET, NEW YORK, NY 10024 Number 3434, 51 pp., 17 ®gures, 13 tables May 14, 2004 New Craniodental Remains of the Quaternary Jamaican Monkey Xenothrix mcgregori (Xenotrichini, Callicebinae, Pitheciidae), with a Reconsideration of the Aotus Hypothesis1 R.D.E. -

Capuchins Are the Most Intelligent New World Monkeys – Perhaps As Intelligent As Chimpanzees
Hooded Capuchin Cebus paella cay Capuchin IQ - Capuchins are the most intelligent New World monkeys – perhaps as intelligent as chimpanzees. They are noted for their ability to fashion and use tools. Because of their ability to learn and remember, they were once trained as “organ grinder” monkeys, are often used in movies and have even been trained to assist disabled people with routine tasks around the house. Get a Grip - Hooded capuchins have a semi-prehensile tail that they can use to grip objects. They have opposable thumbs and big toes on their hands and feet further enabling them to easily manipulate objects. They use their prehensile tail to anchor themselves when climbing around in the trees and to keep them from falling when they sleep. Unlike some monkeys with prehensile tails, capuchins cannot hang by their tail alone. Classification The hooded capuchin is also been referred to as the tufted or brown capuchin. Class: Mammalia Order: Primate Family: Cebidae Genus: Cebus Species: paella Subspecies: cay Distribution This species ranges through northern South America, specifically Venezuela, Brazil, Guyana, Suriname and French Guiana. Habitat Canopies of tropical and mountain rainforests. Physical Description • Hooded capuchins are 12-22 inches (30-56 cm) long with a 15-22 inch (38-56 cm) tail. • Adults weigh six to eight pounds (3-4 kg). • Their fur is light to dark brown with a distinctive black cap on the head. • They have semi-prehensile tails. • Hooded capuchins have tufts of hair that form ridges along the side of the top of their head. Diet What Does It Eat? In the wild: Fruit, flowers, leaves, insects, birds, eggs, lizards, and small mammals. -

The Effects of Habitat Disturbance on the Populations of Geoffroy's Spider Monkeys in the Yucatan Peninsula
The Effects of Habitat Disturbance on the Populations of Geoffroy’s Spider Monkeys in the Yucatan Peninsula PhD thesis Denise Spaan Supervisor: Filippo Aureli Co-supervisor: Gabriel Ramos-Fernández August 2017 Instituto de Neuroetología Universidad Veracruzana 1 For the spider monkeys of the Yucatan Peninsula, and all those dedicated to their conservation. 2 Acknowledgements This thesis turned into the biggest project I have ever attempted and it could not have been completed without the invaluable help and support of countless people and organizations. A huge thank you goes out to my supervisors Drs. Filippo Aureli and Gabriel Ramos- Fernández. Thank you for your guidance, friendship and encouragement, I have learnt so much and truly enjoyed this experience. This thesis would not have been possible without you and I am extremely proud of the results. Additionally, I would like to thank Filippo Aureli for all his help in organizing the logistics of field work. Your constant help and dedication to this project has been inspiring, and kept me pushing forward even when it was not always easy to do so, so thank you very much. I would like to thank Dr. Martha Bonilla for offering me an amazing estancia at the INECOL. Your kind words have encouraged and inspired me throughout the past three years, and have especially helped me to get through the last few months. Thank you! A big thank you to Drs. Colleen Schaffner and Jorge Morales Mavil for all your feedback and ideas over the past three years. Colleen, thank you for helping me to feel at home in Mexico and for all your support! I very much look forward to continue working with all of you in the future! I would like to thank the CONACYT for my PhD scholarship and the Instituto de Neuroetología for logistical, administrative and financial support. -

Stem Members of Platyrrhini Are Distinct from Catarrhines in at Least One Derived Cranial Feature
Journal of Human Evolution 100 (2016) 16e24 Contents lists available at ScienceDirect Journal of Human Evolution journal homepage: www.elsevier.com/locate/jhevol Stem members of Platyrrhini are distinct from catarrhines in at least one derived cranial feature * Ethan L. Fulwood a, , Doug M. Boyer a, Richard F. Kay a, b a Department of Evolutionary Anthropology, Duke University, Box 90383, Durham, NC 27708, USA b Division of Earth and Ocean Sciences, Nicholas School of the Environment, Duke University, Durham, NC 27708, USA article info abstract Article history: The pterion, on the lateral aspect of the cranium, is where the zygomatic, frontal, sphenoid, squamosal, Received 3 August 2015 and parietal bones approach and contact. The configuration of these bones distinguishes New and Old Accepted 2 August 2016 World anthropoids: most extant platyrrhines exhibit contact between the parietal and zygomatic bones, while all known catarrhines exhibit frontal-alisphenoid contact. However, it is thought that early stem- platyrrhines retained the apparently primitive catarrhine condition. Here we re-evaluate the condition of Keywords: key fossil taxa using mCT (micro-computed tomography) imaging. The single known specimen of New World monkeys Tremacebus and an adult cranium of Antillothrix exhibit the typical platyrrhine condition of parietal- Pterion Homunculus zygomatic contact. The same is true of one specimen of Homunculus, while a second specimen has the ‘ ’ Tremacebus catarrhine condition. When these new data are incorporated into an ancestral state reconstruction, they MicroCT support the conclusion that pterion frontal-alisphenoid contact characterized the last common ancestor of crown anthropoids and that contact between the parietal and zygomatic is a synapomorphy of Platyrrhini. -

New Platyrrhine Tali from La Venta, Colombia Department of Anthropology, Northern Illinois University, Dekalb, Illinois 60115
Daniel L. Gebo New platyrrhine tali from La Venta, Colombia Department of Anthropology, Northern Illinois University, DeKalb, Illinois 60115. U.S.A. Two new primate tali were discovered from the middle Miocene of South America at La Venta, Colombia. IGM-KU 8802 is similar in morphology to Callicebus and Aotus, and is allocated to cf. Aotus dindensis, while IGM-KU Marian Dagosto 8803, associated with a dentition of a new cebine primate, is similar to Saimiri. Both tali differ from the other known fossil platyrrhine tali, Departments of Cell, Molecular, and Dolichocebus and Cebupithecia, and increase our knowledge of the locomotor Structural Biology and Anthropology, diversity of the La Venta primate fauna. hrorthrerestern lJniuersi;v, Euanston, Illinois 60208, U.S.A. Alfred L. Rosenberger Department of Anthropology, L’niuersity of Illinois at Chicago, Chicago, Illinois 60680, U.S.A. Takeshi Setoguchi Primate Research Institute, Kyoto C’niniuersiQ,Inuyama Cily, Aichi 484, Japan Received 18 April 1989 Revision received 4 December 1989and accepted 2 1 December 1989 Keywords: Platyrrhini, La Venta, foot bones, locomotion. Journal of Human Evolution (1990) 19,737-746 Introduction Two new platyrrhine tali were discovered at La Venta, Colombia, by the Japanese/American field team working in conjunction with INGEOMINAS (Instituto National de Investigaciones Geologico-Mineras) during the field season of 1988. These two fossils add to the rare but growing number of postcranial remains of extinct platyrrhines from the Miocene of South America (Stirton, 1951; Gebo & Simons, 1987; Anapol & Fleagle, 1988; Ford, 1990). They represent the first new primate postcranials to be described from La Venta in nearly four decades. -

New Primate Genus from the Miocene of Argentina
New primate genus from the Miocene of Argentina Marcelo F. Tejedor*†, Ada´ n A. Tauber‡, Alfred L. Rosenberger§¶, Carl C. Swisher IIIʈ, and Marı´aE. Palacios** *Consejo Nacional de Investigaciones Cientı´ficasy Te´cnicas, Laboratorio de Investigaciones en Evolucio´n y Biodiversidad, Facultad de Ciencias Naturales, Sede Esquel, Universidad Nacional de la Patagonia ‘‘San Juan Bosco,’’ Sarmiento 849, 9200 Esquel, Argentina; ‡Facultad de Ciencias Exactas, Fı´sicasy Naturales, Universidad Nacional de Co´rdoba, Avenida Velez Sarsfield 249, 5000 Co´rdoba, Argentina; §Department of Archaeology and Anthropology, Brooklyn College, City University of New York, 2900 Bedford Avenue, Brooklyn, NY 11210; ¶Division of Vertebrate Zoology (Mammalogy), American Museum of Natural History, Central Park West at 79th Street, New York, NY 11024; ʈDepartment of Geological Sciences, Rutgers University, Piscataway, NJ 08854; and **Museo Regional Provincial ‘‘Padre Manuel Jesu´s Molina,’’ Ramo´n y Cajal 51, 9400 Rı´oGallegos, Argentina Edited by Jeremy A. Sabloff, University of Pennsylvania Museum of Archaeology and Anthropology, Philadelphia, PA, and approved December 28, 2005 (received for review August 4, 2005) Killikaike blakei is a new genus and species of anthropoid from the of the estuary of the Rio Gallegos river. The stratigraphy is late Early Miocene of southeastern Argentina based on the most divided into two members (6): Estancia La Costa (120 m thick pristine fossil platyrrhine skull and dentition known so far. It is part with 18 fossiliferous levels) and Estancia Angelina (103 m thick of the New World platyrrhine clade (Family Cebidae; Subfamily with four fossiliferous levels). There are two types of volcanic ash Cebinae) including modern squirrel (Saimiri) and capuchin mon- at the locality. -

The Size of Major Mammalian Sensory Organs As Measured from Cranial Characters, and Their Relation to the Biology and Evolution of Mammals
HENRY PIHLSTRÖMTheSizeofMammalianSensoryOrgansandTheirRelationtotheBiologyEvolution ofMammals Recent Publications in this Series 24/2012 Anne Vatén Symplastically Transmitted Signals Regulate Pattern Formation during Root Development in Arabidopsis thaliana 25/2012 Lotta Happonen Life on the Edge: Structural Studies of the Extremophilic Viruses P23-77 and STIV2 26/2012 Timo Lehti To Move or to Convene: Regulatory Circuits of Mat Fimbriae in Escherichia coli DISSERTATIONES BIOCENTRI VIIKKI UNIVERSITATIS HELSINGIENSIS 44/2012 27/2012 Sylvie Lefebvre Tumor Necrosis Factors and Chemokines in Hair Development 28/2012 Faraz Ahmad Post-Translational Regulation of KCC2 in the Rat Hippocampus 29/2012 Anne Soikkeli HENRY PIHLSTRÖM Automatable Microplate-Based in vitro Assays for Screening Intestinal Drug Transport and Metabolism 30/2012 Niina Suni Desorption Ionization Mass Spectrometry: Tools for Rapid Bio- and Pharmaceutical Analysis The Size of Major Mammalian Sensory Organs as 31/2012 Pia Saarinen Measured from Cranial Characters, and Their Functional Properties of Visual Pigments Using A1 and A2 Chromophore: From Molecules to Ecology Relation to the Biology and Evolution of Mammals 32/2012 Paula Peltopuro Transcriptional Regulation of GABAergic Neuron Differentiation in the Developing Diencephalon, Midbrain and Anterior Hindbrain 33/2012 Pauli Turunen Studies on OX1 Orexin Receptor Coupling to Arachidonic Acid and Endocannabinoid Signaling 34/2012 Alexandros Kiriazis Synthesis of Six-Membered Rings and Inhibitors of Protein Kinases 35/2012 Jonna Saarimäki-Vire Fibroblast Growth Factor Signaling in the Development of the Midbrain and Anterior Hindbrain 36/2012 Hongbo Zhang UGTs and Glucuronidation Analyses in Caco-2 Cells, Human Microsomes and Recombinant Enzymes 37/2012 Violeta Manole Structural Studies on Viral Receptor-Binding Proteins 38/2012 Anthony Christian Mgbeahuruike Physiological and Molecular Analysis of the Interaction between the Conifer Pathogen, Heterobasidion annosum s.l. -
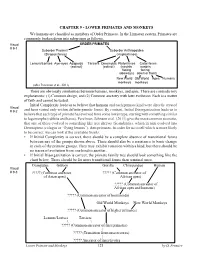
CHAPTER 9 - LOWER PRIMATES and MONKEYS We Humans Are Classified As Members of Order Primates
CHAPTER 9 - LOWER PRIMATES AND MONKEYS We humans are classified as members of Order Primates. In the Linnaean system, Primates are commonly broken down into subgroups as follows. Visual ORDER PRIMATES # 9-1 Suborder Prosimii Suborder Anthropoidea (Strepsirrhines) (Haplorrhines) Lemurs/Lorises Aye-ayes Adapoids Tarsiers Omomyids Platyrrhines Catarrhines (extinct) (extinct) (nostrils nostrils facing facing sideways) down or front) New World Old World Apes Humans monkeys monkeys (after Perelman et al., 2011) There are obviously similarities between humans, monkeys, and apes. There are contradictory explanations: (1) Common design, and (2) Common ancestry with later evolution. Each is a matter of faith and cannot be tested. Initial Complexity leads us to believe that humans and each primate kind were directly created Visual # 9-2 and have varied only within definite genetic limits. By contrast, Initial Disorganization leads us to believe that each type of primate has evolved from some lower type, starting with something similar to lagomorphs (rabbits and hares). Perelman, Johnson et al. (2011) give the most common scenario, that one of these evolved to something like tree shrews (Scandentia), which in turn evolved into Dermoptera (colugos or “flying lemurs”), then primates. In order for us to tell which is more likely to be correct, we can look at the available fossils. • If Initial Complexity is correct, there should be a complete absence of transitional forms between any of the groups shown above. There should also be a resistance to basic change in each of the primate groups. They may exhibit variation within a kind, but there should be no traces of evolution from one kind to another. -

Laboratory Primate Newsletter
LABORATORY PRIMATE NEWSLETTER Vol. 44, No. 1 January 2005 JUDITH E. SCHRIER, EDITOR JAMES S. HARPER, GORDON J. HANKINSON AND LARRY HULSEBOS, ASSOCIATE EDITORS MORRIS L. POVAR, CONSULTING EDITOR ELVA MATHIESEN, ASSISTANT EDITOR ALLAN M. SCHRIER, FOUNDING EDITOR, 1962-1987 Published Quarterly by the Schrier Research Laboratory Psychology Department, Brown University Providence, Rhode Island ISSN 0023-6861 POLICY STATEMENT The Laboratory Primate Newsletter provides a central source of information about nonhuman primates and re- lated matters to scientists who use these animals in their research and those whose work supports such research. The Newsletter (1) provides information on care and breeding of nonhuman primates for laboratory research, (2) dis- seminates general information and news about the world of primate research (such as announcements of meetings, research projects, sources of information, nomenclature changes), (3) helps meet the special research needs of indi- vidual investigators by publishing requests for research material or for information related to specific research prob- lems, and (4) serves the cause of conservation of nonhuman primates by publishing information on that topic. As a rule, research articles or summaries accepted for the Newsletter have some practical implications or provide general information likely to be of interest to investigators in a variety of areas of primate research. However, special con- sideration will be given to articles containing data on primates not conveniently publishable elsewhere. General descriptions of current research projects on primates will also be welcome. The Newsletter appears quarterly and is intended primarily for persons doing research with nonhuman primates. Back issues may be purchased for $5.00 each.