Membrane Transport Anatomy 36 Unit 1 Membrane Transport
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Spring 2013 Lecture 23
CHM333 LECTURES 23: 3/25/13 SPRING 2013 Professor Christine Hrycyna LIPIDS III EFFECT OF CHOLESTEROL ON MEMBRANES: - Bulky rigid molecule - Moderates fluidity of membranes – both increases and decreases o Cholesterol in membranes DECREASES fluidity because it is rigid o Prevents crystallization (making solid) of fatty acyl side chains by fitting between them. Disrupts close packing of fatty acyl chains. Therefore, INCREASED fluidity BIOLOGICAL MEMBRANES CONTAIN PROTEINS AS WELL AS LIPIDS: - Proteins are 20-80% of cell membrane - Rest is lipid or carbohydrate; supramolecular assembly of lipid, protein and carbohydrate - Proteins are also distributed asymmetrically - TWO classes of Membrane Proteins: o Integral Membrane Proteins o Peripheral Membrane Proteins 178 CHM333 LECTURES 23: 3/25/13 SPRING 2013 Professor Christine Hrycyna - INTEGRAL MEMBRANE PROTEINS o Located WITHIN the lipid bilayer o Usually span the bilayer one or more times – called transmembrane (TM) proteins o Hydrophobic amino acids interact with fatty acid chains in the hydrophobic core of the membrane o Can be removed from the membrane with detergents like SDS – need to disrupt the hydrophobic interactions § Membrane Disruption Animation: o http://www.youtube.com/watch?v=AHT37pvcjc0 o Function: § Transporters – moving molecules into or out of cells or cell membranes § Receptors – transmitting signals from outside of the cell to the inside - β Barrel Integral Membrane Proteins § Barrel-shaped membrane protein that is made up of antiparallel β-strands with hydrophilic (interior) and hydrophobic (facing lipid tails). § So far found only in outer membranes of Gram-negative bacteria, cell wall of Gram-positive bacteria, and outer membranes of mitochondria and chloroplasts. 179 CHM333 LECTURES 23: 3/25/13 SPRING 2013 Professor Christine Hrycyna - α-Helical Membrane Proteins - Can cross the membrane once or many times and have multiple transmembrane segments. -

The Membrane
The Membrane Natalie Gugala1*, Stephana J Cherak1 and Raymond J Turner1 1Department of Biological Sciences, University of Calgary, Canada *Corresponding author: RJ Turner, Department of Biological Sciences, University of Calgary, Alberta, Canada, Tel: 1-403-220-4308; Fax: 1-403-289-9311; Email: [email protected] Published Date: February 10, 2016 ABSTRACT and continues to be studied. The biological membrane is comprised of numerous amphiphilic The characterization of the cell membrane has significantly extended over the past century lipids, sterols, proteins, carbohydrates, ions and water molecules that result in two asymmetric polar leaflets, in which the interior is hydrophobic due to the hydrocarbon tails of the lipids. generated a dynamic heterogonous image of the membrane that includes lateral domains and The extension of the Fluid Mosaic Model, first proposed by Singer and Nicolson in 1972, has clusters perpetrated by lipid-lipid, protein-lipid and protein-protein interactions. Proteins found within the membrane, which are generally characterized as either intrinsic or extrinsic, have an array of biological functions vital for cell activity. The primary role of the membrane, among many, is to provide a barrier that conveys both separation and protection, thus maintaining the integrity of the cell. However, depending on the permeability of the membrane several ions are able to move down their concentration gradients. In turn this generates a membrane potential difference between the cytosol, which is found to have an excess negative charge, and surrounding extracellular fluid. Across a biological cell membrane, several potentials can be found. These include the Nernst or equilibrium potential, in which there is no overall flow of a Basicparticular Biochemistry ion and | www.austinpublishinggroup.com/ebooks the Donnan potential, created by an unequal distribution of ions. -

Structure and Activity of Lipid Bilayer Within a Membrane-Protein Transporter
Structure and activity of lipid bilayer within a membrane-protein transporter Weihua Qiua,b,1, Ziao Fuc,1, Guoyan G. Xua, Robert A. Grassuccid, Yan Zhanga, Joachim Frankd,e,2, Wayne A. Hendricksond,f,g,2, and Youzhong Guoa,b,2 aDepartment of Medicinal Chemistry, Virginia Commonwealth University, Richmond, VA 23298; bInstitute for Structural Biology, Drug Discovery and Development, Virginia Commonwealth University, Richmond, VA 23219; cIntegrated Program in Cellular, Molecular, and Biomedical Studies, Columbia University, New York, NY 10032; dDepartment of Biochemistry and Molecular Biophysics, Columbia University, New York, NY 10032; eDepartment of Biological Sciences, Columbia University, New York, NY 10027; fDepartment of Physiology and Cellular Biophysics, Columbia University, New York, NY 10032; and gNew York Structural Biology Center, New York, NY 10027 Contributed by Wayne A. Hendrickson, October 15, 2018 (sent for review July 20, 2018; reviewed by Yifan Cheng and Michael C. Wiener) Membrane proteins function in native cell membranes, but extrac- 1.9 Å (30). Nevertheless, the mechanism of active transport is still tion into isolated particles is needed for many biochemical and far from clear, in part because crucial structural information re- structural analyses. Commonly used detergent-extraction meth- garding protein–lipid interaction is missing (31). The AcrB trimer ods destroy naturally associated lipid bilayers. Here, we devised a has a central cavity between transmembrane (TM) domains of the detergent-free method for preparing cell-membrane nanopar- three protomers, where a portion of lipid bilayer may exist (26). ticles to study the multidrug exporter AcrB, by cryo-EM at 3.2-Å Although detergent molecules and some alkane chains have been resolution. -

Therapeutic Nanobodies Targeting Cell Plasma Membrane Transport Proteins: a High-Risk/High-Gain Endeavor
biomolecules Review Therapeutic Nanobodies Targeting Cell Plasma Membrane Transport Proteins: A High-Risk/High-Gain Endeavor Raf Van Campenhout 1 , Serge Muyldermans 2 , Mathieu Vinken 1,†, Nick Devoogdt 3,† and Timo W.M. De Groof 3,*,† 1 Department of In Vitro Toxicology and Dermato-Cosmetology, Vrije Universiteit Brussel, Laarbeeklaan 103, 1090 Brussels, Belgium; [email protected] (R.V.C.); [email protected] (M.V.) 2 Laboratory of Cellular and Molecular Immunology, Vrije Universiteit Brussel, Pleinlaan 2, 1050 Brussels, Belgium; [email protected] 3 In Vivo Cellular and Molecular Imaging Laboratory, Vrije Universiteit Brussel, Laarbeeklaan 103, 1090 Brussels, Belgium; [email protected] * Correspondence: [email protected]; Tel.: +32-2-6291980 † These authors share equal seniorship. Abstract: Cell plasma membrane proteins are considered as gatekeepers of the cell and play a major role in regulating various processes. Transport proteins constitute a subclass of cell plasma membrane proteins enabling the exchange of molecules and ions between the extracellular environment and the cytosol. A plethora of human pathologies are associated with the altered expression or dysfunction of cell plasma membrane transport proteins, making them interesting therapeutic drug targets. However, the search for therapeutics is challenging, since many drug candidates targeting cell plasma membrane proteins fail in (pre)clinical testing due to inadequate selectivity, specificity, potency or stability. These latter characteristics are met by nanobodies, which potentially renders them eligible therapeutics targeting cell plasma membrane proteins. Therefore, a therapeutic nanobody-based strategy seems a valid approach to target and modulate the activity of cell plasma membrane Citation: Van Campenhout, R.; transport proteins. -

Distinct Endocytic Recycling of Myelin Proteins Promotes Oligodendroglial Membrane Remodeling
834 Research Article Distinct endocytic recycling of myelin proteins promotes oligodendroglial membrane remodeling Christine Winterstein, Jacqueline Trotter and Eva-Maria Krämer-Albers* Department of Biology, Unit of Molecular Cell Biology, University of Mainz, Bentzelweg 3, 55128 Mainz, Germany *Author for correspondence (e-mail: [email protected]) Accepted 20 December 2007 Journal of Cell Science 121, 834-842 Published by The Company of Biologists 2008 doi:10.1242/jcs.022731 Summary The central nervous system myelin sheath is a multilayered proteins were targeted to the late-endosomal/lysosomal specialized membrane with compacted and non-compacted compartment. MOG was also endocytosed by a clathrin- domains of defined protein composition. How oligodendrocytes dependent pathway, but in contrast to MAG, trafficked to the regulate myelin membrane trafficking and establish recycling endosome. Endocytic recycling resulted in the membrane domains during myelination is largely unknown. association of PLP, MAG and MOG with oligodendroglial Oligodendroglial cells respond to neuronal signals by adjusting membrane domains mimicking the biochemical characteristics the relative levels of endocytosis and exocytosis of the major of myelin domains. Our results suggest that endocytic sorting myelin protein, proteolipid protein (PLP). We investigated and recycling of myelin proteins may assist plasma membrane whether endocytic trafficking is common to myelin proteins and remodeling, which is necessary for the morphogenesis of myelin analyzed the endocytic fates of proteins with distinct myelin subdomains. subdomain localization. Interestingly, we found that PLP, myelin-associated glycoprotein (MAG) and myelin- oligodendrocyte glycoprotein (MOG), which localize to compact Supplementary material available online at myelin, periaxonal loops and abaxonal loops, respectively, http://jcs.biologists.org/cgi/content/full/121/6/834/DC1 exhibit distinct endocytic fates. -
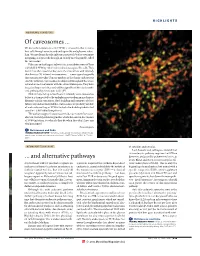
Of Caveosomes
HIGHLIGHTS MEMBRANE TRANSPORT Of caveosomes ... We knew that simian virus 40 (SV40) is unusual in that it enters host cells through caveolae and ends up in the endoplasmic reticu- lum. We now learn that the infectious route of SV40 is even more intriguing, as it proceeds through an entirely novel organelle, called the ‘caveosome’. Pelkmans and colleagues followed the intracellular route of Texas red-labelled SV40 by video-enhanced microscopy of live cells. They found that, after caveolar endocytosis, the virus arrives with relatively slow kinetics (20–40 min) in caveosomes — a new type of organelle that contains caveolin-1 but no markers of the classic endocytic or exocytic pathways. Caveosomes are dispersed throughout the whole cell and are not continuous with the extracellular space. They have irregular shapes and sizes, and, unlike organelles of the classic endo- cytic pathway, they have a non-acidic pH. SV40 remains for up to four hours in relatively static caveosomes before it is transported to the endoplasmic reticulum in much more dynamic tubular containers. Both budding and transport of these tubules depend on microtubules. Caveosomes are probably capable of molecular sorting, as SV40 is included in budding tubules but caveolin-1 is left behind (see picture). The authors suggest that caveosomes do not mature from cave- olae but are independent organelles, which also exist in the absence of SV40 infection. So what do they do when they don’t have any viral passengers? Raluca Gagescu References and links ORIGINAL RESEARCH PAPER Pelkmans, L. et al. Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER. -

The Roles of Lysosomal Exocytosis in Regulated Myelination
Shen YT, Yuan Y, Su WF, Gu Y, Chen G. J Neurol Neuromed (2016) 1(5): 4-8 Neuromedicine www.jneurology.com www.jneurology.com Journal of Neurology & Neuromedicine Mini Review Open Access The Roles of Lysosomal Exocytosis in Regulated Myelination Yun-Tian Shen1, Ying Yuan1,2, Wen-Feng Su1, Yun Gu1, Gang Chen1 1Jiangsu Key Laboratory of Neuroregeneration, Co-innovation Center of Neuroregeneration, Nantong University, Nantong, China 2Affiliated Hospital of Nantong University, Nantong, China ABSTRACT Article Info Article Notes The myelin sheath wraps axons is an intricate process required for Received: June 02, 2016 rapid conduction of nerve impulses, which is formed by two kinds of glial Accepted: July 21, 2016 cells, oligodendrocytes in the central nervous system and Schwann cells in the peripheral nervous system. Myelin biogenesis is a complex and finely *Correspondence: regulated process and accumulating evidence suggests that myelin protein Dr. Gang Chen Jiangsu Key Laboratory of Neuroregeneration Co-innovation synthesis, storage and transportation are key elements of myelination, Center of Neuroregeneration, Nantong University, Nantong, however the mechanisms of regulating myelin protein trafficking are still not China. 226001, Tel: 86-513-85051805, very clear. Recently, the evidences of lysosomal exocytosis in oligodendrocytes Email: [email protected] and Schwann cells are involved in regulated myelination have emerged. In this paper, we briefly summarize how the major myelin-resident protein, as © 2016 Chen G. This article is distributed under the terms of the proteolipid protein in the central nervous system and P0 in the peripheral Creative Commons Attribution 4.0 International License nervous system, transport from lysosome to cell surface to form myelin sheath and focus on the possible mechanisms involved in these processes. -

A. Membrane Functions Biological Membranes Are Composed Of… Membrane Lipid Protein
A. Membrane Functions Biological Membranes are composed of… Membrane Lipid Protein Myelin Sheath 80% 20% Plasma Membrane 50% 50% Mitochondrial 25% 75% Inner Membrane Fig. 5.12: Phospholipids Hydrophilic head 2 Hydrophobic tails Phospholipds are amphipathic molecules (contain both hydrophilic and hydrophobic parts) Phospholipids form Membrane Bilayers Bilayer consisting of two inverted phospholipid layers (leaflets) Hydrophobic ~30 Å ~45 Å Interior (3 nm) (4.5 nm) Hydrophobic interior is an impermeable barrier to passage of hydrophilic molecules, but not to hydrophobic molecules Cholesterol has profound effects on membrane fluidity Fig 7.8: Membrane Fluidity (a) Phospholipid molecules move side-to-side within leaflet easily (lateral diffusion) but do not “flip-flop” across bilayer (transverse diffusion) (b) Phospholipids containing unsaturated acyl chains increase membrane fluidity by reducing packing efficiency (c) Cholesterol reduces membrane fluidity at normal temperatures (reduces phospholipid movement) At low temperatures it keeps membrane fluid (disrupts packing) Membrane Proteins can Move Laterally Within the Lipid Bilayer Membrane proteins labeled with different color fluorescent dyes Supports fluid-mosaic model of a dynamic membrane structure Three Types of Membrane Proteins 1. Integral membrane proteins (transmembrane proteins) Extracellular domain • span the bilayer • transmembrane domain has Transmembrane hydrophobic surface domain • cytosolic and extracellular Cytosolic domains have hydrophilic surfaces domain 2. Lipid-anchored membrane proteins - anchored via a covalently attached lipid 3. Peripheral membrane proteins - interact with hydrophilic lipid head groups or with integral membrane proteins How do proteins cross lipid bilayer membranes? δ- δ+ δ- δ+ Even if the R-groups are hydrophobic, the peptide bond atoms are hydrophilic (polar) and will want to form Hydrogen Bonds; there are no H-bond donors or acceptors in the middle of a lipid bilayer. -

1. Membrane Structure Plasma Membrane
Chapter 5A: Membrane Structure & Function 1. Membrane Structure 2. Diffusion 3. Membrane Transport 1. Membrane Structure Plasma Membrane All cells are enclosed by a plasma membrane • a selectively permeable barrier to “outside” 1 What are Membranes made of? Mainly phospholipids, with some cholesterol (in animal cell membranes) and a variety of membrane proteins. “unsaturated” “saturated” Phospholipids form a Lipid Bilayer • all biological membranes are a lipid bilayer Polar heads face “out” Hydrophobic tails face “in” Phospholipid Bilayer with some Cholesterol and Proteins 2 The Lipid Bilayer is Fluid Phospholipids, cholesterol, membrane proteins can move freely in the bilayer • consistency of the bilayer is like a viscous oil • degree of “fluidity” depends on: 1) temperature 2) types of “fatty tails” (saturated vs unsaturated) 3) amount of cholesterol 2. Diffusion Diffusion Diffusion is the spontaneous movement of a substance from higher to lower concentration • molecules dissolved in liquid move randomly • over time the net effect is equal dispersion of the molecules (provided there is no barrier) • aka “moving down concentration gradient” 3 Osmosis is the Diffusion of Water equal lower higher concentration concentration concentration of solute of solute of solute • water undergoes a net flow from high to low H O solute 2 molecule concentration selectively permeable membrane • has powerful water effects if a molecule barrier is semi- permeable • large molecules can’t diffuse, solute molecule with cluster of water molecules so water keeps diffusing in net flow of water Osmosis can cause cells to shrink, swell in isotonic in hypertonic in hypotonic solution solution solution (equal conc. (more solutes) (less solutes) of solutes) Water will diffuse to where it is less concentrated! 3. -

Secrets of Caveolae- and Lipid Raft-Mediated Endocytosis Revealed by Mammalian Viruses
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Biochimica et Biophysica Acta 1746 (2005) 295 – 304 http://www.elsevier.com/locate/bba Review Secrets of caveolae- and lipid raft-mediated endocytosis revealed by mammalian viruses Lucas Pelkmans Max Planck Institute of Molecular Cell Biology and Genetics, Pfotenhauerstrasse 108, 01307 Dresden, Germany Received 2 May 2005; received in revised form 13 June 2005; accepted 15 June 2005 Available online 5 July 2005 Abstract In recent years, it has been unambiguously shown that caveolae and lipid rafts can internalize cargo upon stimulation by multivalent ligands, demonstrated by the infectious entry routes of certain non-enveloped viruses that bind integrins or glycosphingolipids. We currently understand little about the membrane trafficking principles of this endocytic route, but it is clear that we cannot use paradigms from classical membrane traffic. Recent evidence indicates that caveolae- and lipid raft-mediated endocytosis plays important roles in cell adhesion and anchorage-dependent cell growth, but the underlying mechanisms are not known. In this review, I will introduce new models based on current research that aims at identifying the core machinery, regulatory components and design principles of this endocytic route in order to understand its role in cellular physiology. Again, viruses are proving to be excellent tools to reach that goal. D 2005 Elsevier B.V. All rights reserved. Keywords: Caveolae; Caveosome; Simian Virus 40; Polyoma Virus; Echovirus 1; Endocytosis; Lipid raft; Integrin; Ganglioside; Membrane trafficking 1. Introduction machineries, and therefore to overcome the cell surface barriers, forced them to co-evolve with their hosts as they The plasma membrane is the cell’s interface with the increased in complexity. -

A Saposin-Lipoprotein Nanoparticle System for Membrane Proteins
ARTICLES A saposin-lipoprotein nanoparticle system for membrane proteins Jens Frauenfeld1,10, Robin Löving2, Jean-Paul Armache3, Andreas F-P Sonnen4,5, Fatma Guettou1, Per Moberg1, Lin Zhu6,7, Caroline Jegerschöld6,7, Ali Flayhan8, John A G Briggs4,5, Henrik Garoff2, Christian Löw1,8, Yifan Cheng3,9 & Pär Nordlund1 A limiting factor in membrane protein research is the ability proteins in lipid nanoparticles, such as liposomes and high- to solubilize and stabilize such proteins. Detergents are used density lipoprotein particles6,7 based on apolipoproteins, also most often for solubilizing membrane proteins, but they are termed nanodiscs, provide a potential solution. These approaches associated with protein instability and poor compatibility have been used routinely for biochemical and biophysical studies with structural and biophysical studies. Here we present of membrane proteins and have also been applied for structure a saposin-lipoprotein nanoparticle system, Salipro, which determination by single-particle cryo-EM8–10. However, optimi- allows for the reconstitution of membrane proteins in a zation of the technologies for individual membrane proteins is lipid environment that is stabilized by a scaffold of saposin relatively laborious. proteins. We demonstrate the applicability of the method on Here we present a lipid nanoparticle system that is based on two purified membrane protein complexes as well as by the the saposin protein family. Saposins are known to be modula- direct solubilization and nanoparticle incorporation of a tors of lipid membranes11,12, mostly at an acidic pH within lyso- viral membrane protein complex from the virus membrane. somes. Given their lipid-binding properties, we hypothesized Our approach facilitated high-resolution structural studies of that they could be used to generate a nanoparticle system for the the bacterial peptide transporter PeptTSo2 by single-particle incorporation of membrane proteins (Fig. -

Rho Regulates Membrane Transport in the Endocytic Pathway to Control Plasma Membrane Specialization in Oligodendroglial Cells
3560 • The Journal of Neuroscience, March 28, 2007 • 27(13):3560–3570 Development/Plasticity/Repair Rho Regulates Membrane Transport in the Endocytic Pathway to Control Plasma Membrane Specialization in Oligodendroglial Cells Angelika Kippert,1,2* Katarina Trajkovic,1,2* Lawrence Rajendran,4 Jonas Ries,3 and Mikael Simons1,2 1Centre for Biochemistry and Molecular Cell Biology, University of Go¨ttingen, 37073 Go¨ttingen, Germany, 2Max-Planck-Institute for Experimental Medicine, 37075 Go¨ttingen, Germany, 3Technical University of Dresden, 01062 Dresden, Germany, and 4Max-Planck-Institute of Molecular Cell Biology and Genetics, 01307 Dresden, Germany Differentiation of oligodendrocytes is associated with dramatic changes in plasma membrane structure, culminating in the formation of myelin membrane sheaths. Previous results have provided evidence that regulation of endocytosis may represent a mechanism to control myelin membrane growth. Immature oligodendrocytes have a high rate of clathrin-independent endocytosis for the transport of mem- brane to late endosomes/lysosomes (LE/Ls). After maturation and receiving signals from neurons, endocytosis is reduced and transport of membrane from LE/Ls to the plasma membrane is triggered. Here, we show that changes in Rho GTPase activity are responsible for switching between these two modes of membrane transport. Strikingly, Rho inactivation did not only reduce the transport of cargo to LE/L but also increased the dynamics of LE/L vesicles. Furthermore, we provide evidence that Rho inactivation results in the condensa- tion of the plasma membrane in a polarized manner. In summary, our data reveal a novel role of Rho: to regulate the flow of membrane and to promote changes in cell surface structure and polarity in oligodendroglial cells.