EPISODIC SYNCOPE CAUSED by VENTRICULAR FLUTTER in a TIGER (PANTHERA TIGRIS) Author(S): Daniel M
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Follow-Up of 134 Pediatric Patients with Wolff-Parkinson-White Pattern: Natural Outcome and Medical Treatment
ORIGINAL ARTICLE Follow-up of 134 Pediatric Patients with Wolff-Parkinson-White Pattern: Natural Outcome and Medical Treatment AMALIA N. STEFANI, GABRIELA R. DAL FABBRO, MARÍA J. BOSALEH, ROBERTH VÁSQUEZ, GUSTAVO A. COSTA, RICARDO SPERANZA, JORGE L. GENTILE, CLAUDIO DE ZULOAGAMTSAC Received: 01/03/2013 ABSTRACT Accepted: 01/03/2013 Address for reprints: Objective Amalia N. Stefani The aim of the study was to evaluate the outcome of a pediatric population with Almafuerte 1722 ventricular pre-excitation pattern, supraventricular tachycardia, atrial fibrillation, (1650) San Martín, cardiomyopathies, mortality and medical treatment. Pcia. de Buenos Aires e-mail: [email protected] Methods From 1976 to 2011, a descriptive observational study was conducted on patients with ventricular pre-excitation in the electrocardiogram. All patients underwent an echocardiogram, 101(75.3%) Holter monitoring, and 69 (51.5%) an ergometric test. Radiofrequency ablation was performed in selected patients. Data were expressed as mean and standard deviation. Results The study population consisted of 134 patients; 80 (59.7%) were male. Age at diag- nosis ranged from 2 days to 18 years (mean 6.5±5 years). Clinical follow-up lasted 1 month to 20 years (mean 3.6±3.9 years). Thirty five patients (26.1%) consulted for supraventricular tachycardia, 16 (11.9%) for ventricular pre-excitation, and the remaining 83 patients (61.9%) for other abnormalities. Seventy-six patients (56.7%) had left conduction pathway and 3 patients double conduction pathway. Sixteen pa- tients (11.9%) presented supraventricular tachycardia during follow-up. Overall, 51 patients (38%) had orthodromic tachycardia at 6.3±5.8 years, 10 patients during the neonatal period. -

ICD-9-ICD-10 Codes for Cardiology
ICD-9-ICD-10 Codes for Cardiology ICD-9 Code ICD-10 Code(s) ICD-10 Descrption(s) Atrial Fibrillation and Flutter 427.31, 427.32 I48.0 Paroxysmal atrial fibrillation I48.1 Persistent atrial fibrillation I48.2 Chronic atrial fibrillation I48.3 Typical atrial flutter I48.4 Atypical atrial flutter I48.91* Unspecified atrial fibrillation I48.92* Unspecified atrial flutter Cardiac Arrhythmias (Other) 427.41, 427.42, 427.60, 427.61, 427.69, 427.81, 427.89, 427.9 I49.01 Ventricular fibrillation I49.02 Ventricular flutter I49.1 Atrial premature depolarization I49.2 Junctional premature depolarization I49.3 Ventricular premature depolarization I49.40 Unspecified premature depolarization I49.49 Other premature depolarization I49.5 Sick sinus syndrome I49.8 Other specified cardiac arrhythmias I49.9* Cardiac arrhythmia, unspecified Chest Pain 411.1, 413.1, 413.9, 786.50 to 786.59 range I20.0 Unstable angina I20.1 Angina pectoris with documented spasm I20.8 Other forms of angina pectoris I20.9 Angina pectoris, unspecified R07.1 Chest pain on breathing R07.2 Precordial pain R07.81 Pleurodynia R07.82 Intercostal pain R07.89 Other chest pain R07.9* Chest pain, unspecified Heart Failure 428.0, 428.1, 428.20 to 428.23 range, 428.30 to 428.33 range, 428.40 to 428.43 range, 428.9 I50.1 Left ventricular failure I50.20* Unspecified systolic (congestive) heart failure I50.21 Acute systolic (congestive) heart failure I50.22 Chronic systolic (congestive) heart failure I50.23 Acute on chronic systolic (congestive) heart failure I50.30* Unspecified diastolic (congestive) -

Pub 100-04 Medicare Claims Processing Centers for Medicare & Medicaid Services (CMS) Transmittal 3054 Date: August 29, 2014 Change Request 8803
Department of Health & CMS Manual System Human Services (DHHS) Pub 100-04 Medicare Claims Processing Centers for Medicare & Medicaid Services (CMS) Transmittal 3054 Date: August 29, 2014 Change Request 8803 SUBJECT: Ventricular Assist Devices for Bridge-to-Transplant and Destination Therapy I. SUMMARY OF CHANGES: This Change Request (CR) is effective for claims with dates of service on and after October 30, 2013; contractors shall pay claims for Ventricular Assist Devices as destination therapy using the criteria in Pub. 100-03, part 1, section 20.9.1, and Pub. 100-04, Chapter 32, sec. 320. EFFECTIVE DATE: October 30, 2013 *Unless otherwise specified, the effective date is the date of service. IMPLEMENTATION DATE: September 30, 2014 Disclaimer for manual changes only: The revision date and transmittal number apply only to red italicized material. Any other material was previously published and remains unchanged. However, if this revision contains a table of contents, you will receive the new/revised information only, and not the entire table of contents. II. CHANGES IN MANUAL INSTRUCTIONS: (N/A if manual is not updated) R=REVISED, N=NEW, D=DELETED-Only One Per Row. R/N/D CHAPTER / SECTION / SUBSECTION / TITLE D 3/90.2.1/Artifiical Hearts and Related Devices R 32/Table of Contents N 32/320/Artificial Hearts and Related Devices N 32/320.1/Coding Requirements for Furnished Before May 1, 2008 N 32/320.2/Coding Requirements for Furnished After May 1, 2008 N 32/320.3/ Ventricular Assist Devices N 32/320.3.1/Postcardiotomy N 32/320.3.2/Bridge-To -Transplantation (BTT) N 32/320.3.3/Destination Therapy (DT) N 32/320.3.4/ Other N 32/320.4/ Replacement Accessories and Supplies for External Ventricular Assist Devices or Any Ventricular Assist Device (VAD) III. -
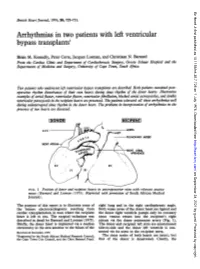
Arrhythmias in Two Patients with Left Ventricular Bypass Transplants'
Br Heart J: first published as 10.1136/hrt.38.7.725 on 1 July 1976. Downloaded from British Heart Journal, 1976, 38, 725-731. Arrhythmias in two patients with left ventricular bypass transplants' Brian M. Kennelly, Peter Corte, Jacques Losman, and Christiaan N. Barnard From the Cardiac Clinic and Department of Cardiothoracic Surgery, Groote Schuur Hospital and the Departments of Medicine and Surgery, University of Cape Town, South Africa Two patients who underwvent left ventricular bypass transplants are described. Both patients sustained post- operative rhythm disturbances of their own hearts during sinus rhythm of the donor hearts. Illustrative examples of atrialflutter, ventricular flutter, ventricular fibrillation, blocked atrial extrasystoles, and double ventricular parasystole in the recipient hearts are presented. The patients tolerated all these arrhythmias well during uninterrupted sinus rhythm in the donor heart. The problems in interpretation of arrhythmias in the presence of two hearts are discussed. [DONOR I IRECIPIENTI Sv C..AOT A ;PULMONARY ARRY http://heart.bmj.com/ RIGHTATRIU_gXARA t,.Y APPENDAGE R.V R.V on September 28, 2021 by guest. Protected copyright. FIG. 1 Position of donor and recipient hearts in anteroposterior view with relevant anasta- moses (Barnard and Losman (1975). Reprinted with permission of South African Medical Journal). The purpose of this report is to illustrate some of right lung and in the right cardiophrenic angle. the bizarre electrocardiograms resulting from Both venae cavae of the donor heart are ligated and cardiac transplantation in man where the recipient the donor right ventricle pumps only its coronary heart is left in situ. The surgical technique was sinus venous return into the recipient's right described in detail by Barnard and Losman (1975). -

Short-Term Exposure to Air Pollution and Cardiac Arrhythmia: a Meta-Analysis and Systematic Review
Int. J. Environ. Res. Public Health 2016, 13, doi:10.3390/ijerph13070642 S1 of S7 Supplementary Materials: Short-Term Exposure to Air Pollution and Cardiac Arrhythmia: A Meta-Analysis and Systematic Review Xuping Song, Yu Liu, Yuling Hu, Xiaoyan Zhao, Jinhui Tian, Guowu Ding and Shigong Wang Figure S1. Flow chart of the literature screening process. Int. J. Environ. Res. Public Health 2016, 13, doi:10.3390/ijerph13070642 S2 of S7 Figure S2. Association between particulate and gaseous components with hospitalization or mortality due to arrhythmia. Int. J. Environ. Res. Public Health 2016, 13, doi:10.3390/ijerph13070642 S3 of S7 Table S1. Search Strategy for PubMed. No. Search Strategy air pollution*/or air pollutant*/or air polluted/or air contamination*/or atmosphere pollution*/or atmosphere pollutant*/ or atmosphere contamination*/or atmospheric pollution*/or atmospheric #1 pollutant*/or atmospheric contamination*/or “particulate matter”/or “PM10”/or “PM2.5”/or ozone/ or “O3”/or “carbon monoxide”/or carbonmonoxide/or “CO“/or “nitrogen dioxide”/or “NO2”/or “sulphur dioxide”/or “sulphur dioxyde”/or “sulfurous anhydride“/or “SO2”.ti,ab. Air Pollution/or Particulate Matter/or Ozone/or Carbon Monoxide/or Nitrogen Dioxide/or Sulfur #2 Dioxide.sh. #3 or/1,2 #4 arrhythmia* /or dysrhythmia* /or “CA”.ti,ab. #5 Arrhythmias, Cardiac.sh. “Sick Sinus Syndrome”/or “SSS”/or “Sick Sinus Node Syndrom”/or Sinus Node Dysfunction*/or #6 Sinus Node Disease*/or Sinus Arrest*.ti,ab. #7 Arrhythmia, Sinus/or Sick Sinus Syndrome/or Sinus Arrest, Cardiac.sh. #8 atrial fibrillation*/or auricular fibrillation*/or “AF".ti,ab. #9 Atrial Fibrillation.sh. -

A Closer Look: Documentation and Coding for Cardiac Conditions
A Closer Look: Documentation and Coding for Cardiac Conditions Heart disease is a broad term used to describe a range of diseases that affect the heart. The various diseases that fall under the umbrella of heart disease include diseases of the heart and blood vessels. The term “heart disease" is often used interchangeably with "cardiovascular disease." Cardiovascular disease generally refers to conditions that involve narrowed or blocked blood vessels that can lead to a heart attack, angina or stroke. Other heart conditions, such as infections and conditions that affect the heart's muscle, valves or beating rhythm are also considered forms of heart disease. All types of heart disease share common traits, but they also have key differences. The goal of this article is to spend some time looking at documentation and diagnosis coding for conditions that fall under the cardiac conditions umbrella to achieve accurate and compliant practices. Dysrhythmias Cardiac dysrhythmia (also known as arrhythmia or irregular heartbeat) is any of a group of conditions in which the electrical activity of the heart is irregular or is faster or slower than normal. The following are some common types of arrhythmia. Tachycardia is an abnormally fast resting heart rate, usually exceeding 100 beats per minute. Supraventricular tachycardia (SVT) is a burst of rapid heartbeats occurring in the top portion of the ventricles. Paroxysmal means the arrhythmia begins and ends suddenly. If the documentation is unclear, the Physician may need to be queried for clarification. Ventricular tachycardia is an abnormal electrical impulse that originates in the ventricles. It may be documented as nonsustained (lasting for less than 30 seconds) or sustained. -

Premature Ventricular Contraction Increases the Risk of Heart Failure
www.nature.com/scientificreports OPEN Premature ventricular contraction increases the risk of heart failure and ventricular tachyarrhythmia Yun Gi Kim1,3, Yun Young Choi1,3, Kyung‑Do Han2, Kyoung Jin Min1, Ha Young Choi1, Jaemin Shim1, Jong‑Il Choi1* & Young‑Hoon Kim1 Premature ventricular contraction (PVC), a common arrhythmia afecting 1–2% of the general population, has been considered to have a benign clinical course. However, people with PVC often develop heart failure and ventricular arrhythmias such as ventricular tachycardia. We aimed to clarify the risk of heart failure and lethal ventricular arrhythmias in people with PVC. The Korean National Health Insurance Service database was used for this study. People who underwent nationwide health check‑ups in 2009 were enrolled in this study and clinical follow‑up data until December 2018 were analyzed. Newly diagnosed PVC in 2009 (≥ 1 inpatient or outpatient claim) were identifed and cumulative incidence of heart failure (≥ 1 inpatient claim) and ventricular arrhythmias (≥ 1 inpatient or outpatient claim) were compared. A total of 4515 people were frst diagnosed with PVC in 2009 among 9,743,582 people without prior history of PVC, heart failure, or ventricular arrhythmias. People with newly diagnosed PVC in 2009 had a signifcantly higher incidence of heart failure compared to those without PVC [adjusted hazard ratio (HR) 1.371; 95% confdence interval (CI) 1.177–1.598; p < 0.001]. Signifcant interaction was observed between age and PVC with young age people at greater risk of developing heart failure for having PVC. The incidence of ventricular arrhythmia was also signifcantly increased in people with PVC (HR 5.588; 95% CI 4.553–6.859; p < 0.001). -

ICD-10: Clinical Concepts for Cardiology
ICD-10 Clinical Concepts for Cardiology ICD-10 Clinical Concepts Series Common Codes Clinical Documentation Tips Clinical Scenarios ICD-10 Clinical Concepts for Cardiology is a feature of Road to 10, a CMS online tool built with physician input. With Road to 10, you can: l Build an ICD-10 action plan customized l Access quick references from CMS and for your practice medical and trade associations l Use interactive case studies to see how l View in-depth webcasts for and by your coding selections compare with your medical professionals peers’ coding To get on the Road to 10 and find out more about ICD-10, visit: cms.gov/ICD10 roadto10.org ICD-10 Compliance Date: October 1, 2015 Official CMS Industry Resources for the ICD-10 Transition www.cms.gov/ICD10 1 Table Of Contents Common Codes • Abnormalities of • Hypertension Heart Rhythm • Nonrheumatic • Atrial Fibrillation and Flutter Valve Disorders • Cardiac Arrhythmias (Other) • Selected Atherosclerosis, • Chest Pain Ischemia, and Infarction • Heart Failure • Syncope and Collapse Clinical Documentation Tips • Acute Myocardial • Atheroclerotic Heart Disease Infraction (AMI) with Angina Pectoris • Hypertension • Cardiomyopathy • Congestive Heart Failure • Heart Valve Disease • Underdosing • Arrythmias/Dysrhythmia Clinical Scenarios • Scenario 1: Hypertension/ • Scenario 4: Subsequent AMI Cardiac Clearance • Scenario: CHF and • Scenario 2: Syncope Pulmonary Embolism Example • Scenario 3: Chest Pain Common Codes ICD-10 Compliance Date: October 1, 2015 Abnormalities of Heart Rhythm (ICD-9-CM 427.81, 427.89, 785.0, 785.1, 785.3) R00.0 Tachycardia, unspecified R00.1 Bradycardia, unspecified R00.2 Palpitations R00.8 Other abnormalities of heart beat R00.9* Unspecified abnormalities of heart beat *Codes with a greater degree of specificity should be considered first. -

ECG Made Easy Part 2 – ECG Quiz
ECG made easy Part 2 – ECG Quiz • Presented by: • Dr Randall Hendriks, Interventional Cardiologist – Western Australia 1 ? Axis 1. Left 2. Right 3. Indeterminate ? Axis 1. Left 2. Right 3. Indeterminate ? Axis 1. Left 2. Right 3. Indeterminate Page 4 ? Axis 1. Left 2. Right 3. Indeterminate Page 5 ? Axis 1. Left 2. Right 3. Indeterminate Page 6 ? Axis 1. Left 2. Right 3. Indeterminate Standard limb lead reversal! Page 7 Tachycardia 1. Atrial fibrillation 2. Atrial flutter 3. SVT 4. Sinus tachycardia 5. Junctional Page 8 Tachycardia 1. Atrial fibrillation 2. Atrial flutter 3. SVT 4. Sinus tachycardia 5. Junctional Page 9 Tachycardia 1. Atrial fibrillation 2. Atrial flutter 3. SVT 4. Sinus tachycardia 5. Junctional Page 10 Tachycardia 1. Atrial fibrillation 2. Atrial flutter 3. SVT 4. Sinus tachycardia 5. Junctional Page 11 Tachycardia 1. Atrial fibrillation 2. Atrial flutter 3. SVT 4. Sinus tachycardia 5. Junctional Page 12 Tachycardia 1. Atrial fibrillation 2. Atrial flutter 3. SVT 4. Sinus tachycardia 5. Junctional Page 13 Tachycardia 1. Atrial fibrillation 2. Atrial flutter 3. SVT 4. Sinus tachycardia 5. Junctional Page 14 Tachycardia 1. Atrial fibrillation 2. Atrial flutter 3. SVT 4. Sinus tachycardia 5. Junctional Page 15 Tachycardia 1. Atrial flutter 2. Atrial fibrillation 3. SVT 4. Sinus tachycardia 5. Junctional Page 16 Tachycardia 1. Atrial flutter 2. Atrial fibrillation 3. SVT 4. Sinus tachycardia 5. Junctional Page 17 Bradycardia 1. Sinus bradycardia 2. First degree AV block 3. Mobitz Type I (Wenckebach) 4. Mobitz Type 2 5. Complete heart block Page 18 Bradycardia 1. Sinus bradycardia 2. -

Middle-Aged Woman with Delayed Diagnosis of Severe Mitro-Aortic Valvular Rheumatic Heart Disease Following Tachyarrhythmic Event
Middle-aged woman with delayed diagnosis of severe mitro-aortic Valvular rheumatic heart disease following tachyarrhythmic event Mulher de média idade com diagnóstico tardio de valvulopatia mitro-aórtica remumática Andrés Ricardo Pérez-Riera M.D. Ph.D. Physician of Hospital do Coração (HCor) - Sao Paulo-Brazil In charge of Electro-vectorcardiogram sector – Cardiology Discipline –ABC Faculty of Medicine –ABC Foundation - Santo André –São Paulo – Brazil. [email protected] Case report A 41-year-old Caucasian woman, housewife, natural of the countryside of São Paulo Brazil, from a low socioeconomic background presented in our clinical consultation after internment 2 Month ago consequence of tachyarrithtymic event At this time, her only symptom was intermittent palpitations. She had no recollection of previous rheumatic fever. History of Present Illness (HPI) She refer the last Month presented fatigue, especially during times of increased physical activity, progressive shortness of breath especially with exertion or when lie down a dry cough presented at rest and unique fast regular palpitation episode that its motives emergency internment and intra-hospitalar treatment with electric cardioversion and use of intravenous drugs The patient denied fever, chest pain, or other systemic symptoms. She has no recollection of any previous symptoms or antecedent of repetitive streptococcal throat infections, murmurs or joints pain in the pass. There was no personal or familial history of cardiovascular disease or rheumatic fever picture. Physical She was breathing well, acianotic, without fever and cored. She was in sinus rhythm, BP: normal systolic blood pressure and low diastolic=140/30-0 mmHg with Watson's water hammer pulse. -

Supraventricular Tachycardia: Diagnosis and Current Acute Management
Archives ofDisease in Childhood 1991; 66: 647-652 647 PERSONAL PRACTICE Arch Dis Child: first published as 10.1136/adc.66.5.647 on 1 May 1991. Downloaded from Supraventricular tachycardia: diagnosis and current acute management Janice A Till, Elliot A Shinebourne There is a small but potentially avoidable inci- dence of death associated with childhood supra- ventricular tachycardia.1 2 The time of greatest risk is when the child presents for the first time and requires therapeutic intervention. Infants and children tolerate arrhythmias less well than adults as they are more dependent on heart rate for cardiac output and have less reserve.3 4 They are also at risk from drugs given as treatment.5 We discuss our current acute man- agement of supraventricular tachycardia. Diagnosis Between 30 and 40% of children who present with supraventricular tachycardia do so within the first few weeks of life. Their presentation is variable. Supraventricular tachycardia can be the cause of unexplained hydrops of the fetus or Figure I An electrocardiogram recordedfrom a I week old can result in sudden profound cardiovascular neonate with atrioventricular re-entry tachycardia showing collapse in the newborn period.6 More usually the typicalpattern with a P' wave almost midway between neonates and small infants will present with QRS complexes. symptoms of increasing tachypnoea, poor feed- http://adc.bmj.com/ ing, and pallor which have developed over a few days. Occasionally supraventricular tachycardia is intermittent and a strong index of suspicion Classification of supraventricular tachycardia must be maintained if the diagnosis is not to be Junctional tachycardias missed. -

Spontaneously Terminating Ventricular Fibrillation and Asystole Induced By
86 Heart 1998;80:86–88 CASE REPORT Heart: first published as 10.1136/hrt.80.1.86 on 1 July 1998. Downloaded from Spontaneously terminating ventricular fibrillation and asystole induced by silent ischaemia causing recurrent syncope M U A Mustafa, C S R Baker, J D Stephens Abstract ing percutaneous transluminal angioplasty A 57 year old man was admitted for inves- and stenting. The patient has had no tigation of recurrent syncopal attacks. further syncopal attacks. Holter monitoring during an attack while (Heart 1998;80:86–88) in hospital revealed a unique sequence of gross ST segment elevation, ventricular Keywords: ventricular fibrillation; Holter monitoring; tachycardia, prolonged ventricular fibril- coronary stenoses; silent ischaemia; arrhythmias lation, asystole, junctional and ventricular escape rhythm, and finally spontaneous restoration of sinus rhythm with severe ST A 57 year old man presented to the accident segment depression. Subsequent coronary and emergency department following a synco- arteriography demonstrated severe sten- pal attack. There was no loss of bladder oses of the right coronary artery, prompt- control, tongue biting or seizure. There was no http://heart.bmj.com/ on September 28, 2021 by guest. Protected copyright. Cardiology Department, Queen Mary Block, Old Church Hospital, Romford RM7 0BE, UK M U A Mustafa J D Stephens Cardiology Department, Hammersmith Hospital, Du Cane Road, London W12 0NN, UK C S R Baker Correspondence to: Dr Mustafa. Accepted for publication 9 March 1998 Spontaneously terminating VF and asystole causing recurrent syncope 87 history of angina-like chest pain. A complete blood pressure was 130/75 mm Hg. Cardiovas- physical examination and initial routine inves- cular, respiratory, gastrointestinal, and central tigations were normal.