Basil: a Source of Aroma Compounds and a Popular Culinary and Ornamental Herb*
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Crop Profile for Basil in New Jersey Ocimum Basilicum (L.) O
Crop Profile for Basil in New Jersey Ocimum basilicum (L.) O. tenuiflorum O. americanum Lamiales: Lamiaceae Prepared: 2008 General Production Information Yearly production: Approximately 450 acres % of crop for fresh market: 100% % of crop grown for retail market: 10 % % of crop grown for wholesale market: 90 % Production Regions The majority of basil production occurs in the southern New Jersey counties of Atlantic (100 acres), Cumberland (225 acres), and Gloucester (50 acres). There is some basil production in the central counties of Monmouth, Ocean, and Burlington, amounting to less than 50 acres. Soils in southern and much of central New Jersey are light, ranging from sand to sandy loam with some areas of silt loam. Basil production in the southern region extends from early April to October. In this region, basil is predominantly grown for the wholesale market, with plant stems generally cut or pulled, washed and bundled in 12-15 plant or stem bunches. Wholesale units are 15-count (bunch) crates. Some basil in this region is produced for the retail market and sold at farm stands or directly to restaurants. In this case, basil is generally sold by the bunch. In the northern counties of Hunterdon, Mercer, Morris, Warren, and Sussex, many growers produce basil, but on minimal acreage. Soils in the northern region are typically Piedmont (heavy silt loams) and Appalachian (shaley) soils. The field season begins in mid-May here, and continues until cold weather terminates production. In the north, basil is grown exclusively for retail at farm stands and at community-sponsored farmers markets. Typical growers in this region produce less than one half acre of basil. -

The Vascular Plants of Massachusetts
The Vascular Plants of Massachusetts: The Vascular Plants of Massachusetts: A County Checklist • First Revision Melissa Dow Cullina, Bryan Connolly, Bruce Sorrie and Paul Somers Somers Bruce Sorrie and Paul Connolly, Bryan Cullina, Melissa Dow Revision • First A County Checklist Plants of Massachusetts: Vascular The A County Checklist First Revision Melissa Dow Cullina, Bryan Connolly, Bruce Sorrie and Paul Somers Massachusetts Natural Heritage & Endangered Species Program Massachusetts Division of Fisheries and Wildlife Natural Heritage & Endangered Species Program The Natural Heritage & Endangered Species Program (NHESP), part of the Massachusetts Division of Fisheries and Wildlife, is one of the programs forming the Natural Heritage network. NHESP is responsible for the conservation and protection of hundreds of species that are not hunted, fished, trapped, or commercially harvested in the state. The Program's highest priority is protecting the 176 species of vertebrate and invertebrate animals and 259 species of native plants that are officially listed as Endangered, Threatened or of Special Concern in Massachusetts. Endangered species conservation in Massachusetts depends on you! A major source of funding for the protection of rare and endangered species comes from voluntary donations on state income tax forms. Contributions go to the Natural Heritage & Endangered Species Fund, which provides a portion of the operating budget for the Natural Heritage & Endangered Species Program. NHESP protects rare species through biological inventory, -

FAMILY PHYSICIAN with Essential Oils “I Finally Figured out How to Use Essential Oils—TAKE OFF the CAP”
FAMILY PHYSICIAN With Essential Oils “I finally figured out how to use essential oils—TAKE OFF THE CAP” You can use all of the oils 3 ways; Topically, Internally (drip into an empty gel cap) and with the diffuser. If the bottle has a ‘supplement’ box on the label, you can feel completely safe using it internally. When diluting* the essential oils, use one drop of Coconut Oil for one or two drops of the essential oil. It is not necessary to dilute the essential oils, but for some essential oils which are particularly cool (Peppermint, Wintergreen) or hot (Oregano, Cinnamon, Clove, Cassia), it may not be comfortable on your skin without diluting, and may be particularly uncomfortable for a child. If you use an oil without diluting and it is uncomfortable to the recipient, simply apply a small amount of coconut oil and it will quickly resolve the problem. Also, if you are using the oils on an open sore or wound, it is always a good idea to dilute with coconut oil. Diffusion is powerful because the child can breathe it in and it kills microorganisms in the air which helps stop the spread of sickness. If the oils get in the eyes, it will sting but will not do damage. Simply rub a few drops of your diluter oil on the eye and it will help to relieve the suffering. You can combine and mix any of the oils as much as you like and the oils are safe with any medication. If you are not sure how to apply the oil, know that you can ALWAYS rub it on the bottoms of the feet and you will get the full affect. -
Herbs Such As Spearmint
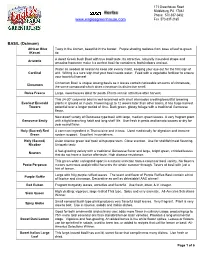
171 Greenhouse Road Middleburg, PA 17842 Phone: 570-837-0432 www.englesgreenhouse.com Fax 570-837-2165 BASIL (Ocimum) African Blue Tasty in the kitchen, beautiful in the border. Purple shading radiates from base of leaf to green (Kasar) tip. A dwarf Greek bush Basil with true basil taste. Its attractive, naturally mounded shape and Aristotle amazing fragrance make it a perfect basil for containers, both indoors and out. Water as needed all season to keep soil evenly moist, keeping your eye out for the first sign of Cardinal wilt. Wilting is a sure sign that your basil needs water. Feed with a vegetable fertilizer to ensure your bountiful harvest Cinnamon Basil is unique among basils as it leaves contain noticeable amounts of cinnamate, Cinnamon the same compound which gives cinnamon its distinctive smell. Dolce Fresca Large, sweet leaves ideal for pesto. Plants remain attractive after harvest. This 24-30” columnar basil is well-branched with short internodes creating beautiful towering Everleaf Emerald plants in ground or in pots. Flowering up to 12 weeks later than other basils, it has huge harvest Towers potential over a longer period of time. Dark green, glossy foliage with a traditional Genovese flavor. New dwarf variety of Genovese type basil with large, medium green leaves. A very fragrant plant Genovese Emily with a tight branching habit and long shelf life. Use fresh in pesto and tomato sauces or dry for year round flavor. Holy (Sacred) Red A common ingredient in Thai cuisine and in teas. Used medicinally for digestion and immune Green system support. -

Herbaceous: Six Important Flavors in Herbs
Herbaceous: Six Important Flavors in Herbs 1. Anethole (the taste of anise or licorice, ouzo, pernod, sambuca): tarragon and fennel have anethole, and even basil has a mild anethole flavor. Thai basil has a strong anethole taste. The licorice herbs are the most food friendly herbs available. Use licorice herbs, and your food—especially any fish or seafood dish—will sparkle. These anise-tasting herbs are also spectacular on eggs, cheese, meat, and tomatoes. French tarragon (a different taste than Russian tarragon), is the flavor found in béarnaise sauce. http://www.epicurious.com/recipes/food/views/bearnaise-sauce- 395049 2. Lemon tasting herbs: lemongrass, lemon verbena, lemon thyme, and sorrel. It’s easy to add lemon to nearly any dish. So make sure than your herb garden contains at least one of the lemon-tasting herbs. 3. Resinous, herbs such as rosemary, thyme, and sage, have a resin, or woody, quality. The flavor of resinous herbs comes through in cooking and is a wonderful match for meats. But be careful. That taste can overpower: the longer rosemary cooks, the stronger the flavor gets. Sage and thyme are very easy to grow and will come back with vigor next year. The flavor of sage is also quite musky in taste and aroma, which is why it blends so well with beans and meats. 4. Grassy: such as dill and parsley. Don’t overlook the value of parsley. It is very herbaceous, which offers a balance to other flavors on the plate. Parsley has a very mild anethole taste. For example, Italians use a garnish called gremolati, where the heat of raw garlic is blended with the sour of lemon zest and balanced with a whole bunch of grassy parsley. -

Vitro Determination of Sun Protection Factor of Ocimum Basilicum, Linn
International Journal of Pharmacy and Pharmaceutical Sciences ISSN- 0975-1491 Vol 2, Suppl 4, 2010 Research Article FORMULATION AND IN VITRO DETERMINATION OF SUN PROTECTION FACTOR OF OCIMUM BASILICUM, LINN. LEAF OILS SUNSCREEN CREAM SHANTANU KALE, AMOL SONAWANE*, AMMAR ANSARI, PRASHANT GHOGE, ASHWINI WAJE Department of Pharmacognosy, MGV’s Pharmacy College, MumbaiAgra Highway, Panchvati. Nashik 422003 Received: 12 July 2010, Revised and Accepted: 23 August 2010 ABSTRACT The increasing awareness of the risk of skin cancer with the sun exposure requires that sunscreen products be approximately tested and labeled. Although there exists information on the possible photon‐induced reaction such as photoirritation and photosensitization produced by sunscreen creams containing synthetic photo‐protective chemicals. Effect of sunscreen cream depends upon sun protection factor (SPF) value and substantivity. In addition due to high cost and time consumption of in vivo SPF determination methodologies, in vitro SPF determination is gaining more importance. The aim of present study was to formulate a sunscreen cream containing essential oil of Ocimum basilicum Linn. as an active ingredient and evaluate experimental method for in vitro SPF determination. Keywords: Ocimum basilicum, UV Protection, Sun protection factor, Sunscreens INTRODUCTION Ocimum basilicum, Linn. (basil) belongs to the plant family Lamiaceae, sub‐family Nepetoideae, and the genus sensulato10. Plant Sunlight composed of various wavelengths ranging from commonly known as sweet or garden basil (Engl.), Munjariki (Sans.), ultraviolet light through infrared to visible light. Exposure to solar basilikumkraut (Ger.), Herbe de grand basilic (Fr.), Common radiation is recognized to have negative effects on the human skin. basil11,12. Lamiaceae plants are native through the subtropics, Among all, ultraviolet light is the most harmful to the skin and especially throughout the Mediterranean region. -

Morphological Characteristics and Susceptibility of Basil Species and Cultivars to Peronospora Belbahrii
DISEASE AND PEST MANAGEMENT HORTSCIENCE 51(11):1389–1396. 2016. doi: 10.21273/HORTSCI09778-16 P. Belbahrii, was first reported in Uganda in 1932 as Peronospora sp. and later in 1937 as Peronospora lamii (Hansford, 1933, Morphological Characteristics and 1938). BDM was not reported again until 2001 in Switzerland (Heller and Baroffio, Susceptibility of Basil Species and 2003). After this initial confirmation, other countries throughout Europe, the Mediter- Cultivars to Peronospora belbahrii ranean, and continents across the world reported BDM for the first time (Belbahri Kathryn Homa et al., 2005; Kofoet et al., 2008; Lefort et al., Department of Plant Biology and Pathology, Rutgers University, Foran Hall, 59 2008; McGrath, 2011). This pathogen was Dudley Road, New Brunswick, NJ 08901-8520; and IR-4 Project Headquarters, first reported in the United States in 2007 in southern Florida (Roberts et al., 2009). Rutgers University, 500 College Road East, Suite 201W, Princeton 08540 Since then, the disease has spread across the William P. Barney continental United States and Hawaii (Wye- nandt et al., 2015). Although the epidemiol- IR-4 Project Headquarters, Rutgers University, 500 College Road East, Suite ogy of the pathogen is not completely 201W, Princeton 08540 understood, BDM appears to have been spread globally via infested seed as well as Daniel L. Ward and Christian A. Wyenandt through wind currents (Thines et al., 2009; Department of Plant Biology and Pathology, Rutgers University, Rutgers Wyenandt et al., 2015). Agricultural Research and Extension Center, 121 Northville Road, Over 5000 ha of sweet basil is grown in Bridgeton, NJ 08302 the United States on an annual basis (J.E. -

The Edible Garden Shopping List
THE EDIBLE GARDEN SHOPPING LIST BOTANICAL NAME COMMON NAME PRICE QUANTITY TOTAL COST Abelmoschus esculentus 'Clemson Okra $5 Spineless' Allium ampeloprasum 'American Leek $5/4 pack Flag' Allium schoenoprasum Chives $5/4 pack Alyosia tryphylia Lemon Verbena $6 Anethum graveolens 'Hera' Dill $5/4 pack Artemesia dracunculus French Tarragon $5 Beta vulgaris 'Bright Lights' Swiss Chard $5 Borago officinalis Borage $5/4 pack Brassica oleracea ‘Flash’ Collards $5 Brassica oleracea Lacinato Kale $5 Brassica oleracea 'Long Island’ Brussels Sprouts $5 Brassica oleracea Red Russian Kale $5 Brassica rapa chinensis Toy Choy Chinese White $5/4 pack Cabbage (Pak Choi) Capsicum annuum Poblano Poblano Pepper $5 Capsicum annuum 'Ace' Bell Pepper $5 Capsicum annuum ‘Biquinho’ Little Bird Hot Pepper $5 Capsicum annuum 'California Bell Pepper $5 Wonder' Capsicum annuum ‘Cornito Giallo’ Bulls Horn Pepper $5 Capsicum annuum Habenero Habenero Pepper $5 Capsicum annuum 'Jalapeno Early Jalapeno Early Hot Pepper $5 Hot' Capsicum annuum 'Mellow Star' Mellow Star Shishito $5 Pepper Citrullus lanatus ‘Cal Sweet’ Cal Sweet Watermelon $5 Citrus limon x medica Ponderosa Lemon $6 Coriandrum sativum Cilantro $5 Cucumis melo 'Sugar Cube’ Cucumber $5 Cucumis sativus 'Diva' Cucumber $5 Cucumis sativus 'Northern Pickling' Northern Pickling $5 Cucumber Cucumis sativus 'Salad Bush' Salad Bush Cucumber $5 Cucurbita pepo 'Early Butternut' Butternut Squash $5 Cucurbita pepo ‘Honey Bear’ Acorn Squash $5 Cucurbita pepo 'Spineless Beauty' Zucchini $5 Cucurbita pepo ‘Tempest’ Summer -

Patio Menu Dessert
PATIO MENU warm marinated olives $5 gf v pickle plate seasonal vegetables, mustard seed $5 gf v bread plate sourdough, cultured butter $6 vg crispy potatoes parmigiano-reggiano, chili aioli $7 vg gf chilled beet soup preserved lemon yogurt, lemon basil, sumac $8 vg gf roasted cabbage calabrian chili butter $8 vg gf roasted weiss farm zucchini balsamic, flowering coriander $8 vg gf df burrata beets, opal basil, cherries, wood sorrel $10 vg summer vegetable salad sherry vinaigrette $11 v gf chicken liver pate rhubarb jam, bronze fennel, crispy chicken skin, sourdough toast $11 caesar oles farm kale, sourdough crumbs, pecorino, fennel $11 vg smoked sockeye rillette creme fraiche, sour cherries, organic dill, toasted sourdough rye $13 hummus za’atar bread, pickled veg, toasted seeds $13 v potato gnocchi beet green pistachio pesto, fava beans, pecorino $16 house ground cheeseburger 3 year grafton cheddar, chili aioli, brioche bun, pickles, crispy potatoes $20 seared scallops corn, fennel, cherry tomatoes, scallion, shiso $30 gf roasted erbe verde ½ chicken fingerlings, labneh, za’atar, oles swiss chard, fresh picked mint $32 gf 16oz niman ranch strip steak yellow beans, walla wallas, truffled pecorino, cherry tomatoes, roasted onion jus $52 gf sourdough pizza & vino special naturally leavened, swiss chard pesto, truffled pecorino & zucchini with your bottle of choice: prosecco, sauvignon blanc or pinot noir $29 DESSERT raspberry lime sorbet $6 gf v chocolate semifreddo horchata ice cream, waffle cone, mezcal infused cherries, toasted almonds -

The Rose Care Guide
Common Herbs & Their Uses Provided by Mid City Nursery BASIL (Ocimum basilicum): Varieties -Sweet Basil (large leaf). Bush Basil (small leaf), Lemon Basil (lemon scented): Annuals that grow to 24". Full sun. Plant 12" apart. Strong, sweet minty taste & aroma. USES: Goes well with tomatoes, cheese, poultry, eggs, & vegetables. Used in pizza, pesto, Italian, French, & Greek dishes. Add late in cooking. GOES WITH: Bay, chives, dill, garlic, marjoram, oregano, parsley, savory, & thyme. BURNET (Salad Burnet) (Poterium sanguisorba)- Perennial growing to 8" first year, up to 18". Plant 12" apart. Likes 6 hours of sun. Nutty, cucumber-like flavor, does not upset stomach like cucumber, use young leaves. USE IN: Salads, cream or cottage cheese, vinegars. GOES WITH: Chervil, parsley, rosemary, tarragon. CHAMOMILE (Matricaria recutita) -Annual. Sun. Plant 4" to 6" apart. USES: Tea from fragrant leaves or from dried flower heads. (This is the tea Peter Rabbit drank). Leaves or flowers for scented sachets. CHIVES (Allimum schoenoprasum) - Perennial 12". Sun. Plant in clumps. Mild onion flavor, thrives on being cut back to base. USES: Herb butters, potatoes, vegetables, eggs, fish, soups, salads, sauces. Need little cooking. Flowers are edible. GOES WITH: Basil, cilantro, cress, dill, lemon balm, marjoram, oregano, parsley, sorrel, tarragon, thyme. GARLIC CHIVES -Same as chives with mild, garlic flavor. CORIANDER or CILANTRO (Coriandrum sativum) -Annual to 18". Sun. Bolts in heat, plant 6" to 8" apart. Produces Coriander seeds used in baking. Leaves of the plant are known as cilantro and resemble parsley foliage with bittersweet orange-sage flavor. USES: Mexican dishes. Use as a topping with hot peppers, tomatoes, chicken, seafood, beef. -

Table E-1. Vegetation Species Found on Wake Atoll
Table E-1. Vegetation Species Found on Wake Atoll Scientific Name Common Name Abutilon albescens Sweet monkeybush Abutilon asiaticum var. albescens Indian mallow Agave americana American century plant Agave angustifolia century plant Agave sisalana Sisal Agave sp. agave sp. Aglaonema commutatum Aglaonema Allium cepa Onion Allium fistulosum Green onion Allium sp. Onion sp. Allium tuberosum Chinese chive Aloe vera Aloe Alpinia galanga Greater galangal Alpinia purpurata Pink ginger; Jungle Queen Amaranthus dubius Spleen amaranth Amaranthus graecizans Tumbleweed Amaranthus tricolor Joseph′s coat Amaranthus viridis Slender amaranth Ananas comosus Pineapple Anethum graveolens Dill Annona muricata Soursop Annona squamosa Sweetsop Apium petroselinum Garden parsley Araucaria heterophylla Norfolk Island pine Asparagus densiflorus Sprenger asparagus fern Asplenium nidus Bird’s-nest fern Barringtonia asiatica Fish poison tree Bauhinia sp. Camel’s foot tree Bidens alba white beggar-ticks Bidens pilosa var. minor Beggar-ticks Boerhavia albiflora var. powelliae -- Boerhavia diffusa Red Spiderling Boerhavia repens anena Boerhavia sp. Spiderling sp. Bothriochloa pertusa Indian blue grass Bougainvillea spectabilis bougainvillea Brassica nigra Mustard Brassica oleracea var. italica Brocolli Caesalpinia bonduc Grey nickers Caladium bicolor Caladium Calotropis gigantea Crown flower Capsicum frutescens Cayenne pepper Capsicum annuum chili pepper Table E-1. Vegetation Species Found on Wake Atoll Scientific Name Common Name Carica papaya Papaya Casuarina equisetifolia -

Pigeon Pea Hummus Pineapple and Mango
PIGEON PEA HUMMUS WITH GRILLED INGREDIENTS DIRECTIONS PINEAPPLE AND MANGO For Hummus Hummus 3 cups cooked drained pigeon peas 1. Add pigeon peas in the food processor and mix until it SALAD 1 1/3 cup of light roasted tahini has a paste consistency. 5 confit or roasted garlic cloves 2. With the machine running add the tahini, roasted or 4 tablespoons fresh lemon juice confit garlic and lemon juice. lemon zest 3. Finally add slowly the iced water until you get a smooth 1 cup of ice water Xavier Pacheco and creamy paste, adjust salt as needed. salt to taste Chef and Owner of La Jaquita Baya Restaurant 4. Transfer to a glass bowl and cover with plastic. REALITY ADELANTE For Grilled Pineapple and Mango Salad 5. Refrigerate if needed. 1 cup sliced and grilled pineapples cut in cubes 1 cup mango slices cut in cubes Grilled Pineapple and Mango Salad ½ cup grape tomatoes in halves 1. Mix fruits and leaves in a mixing bowl. ½ cucumber cut in cubes 2. Add lemon juice to taste and season with sumac and salt. ½ cup Mediterranean parsley leaves or long leave parsley ½ cup cilantro leaves Plating ½ cup lemon basil leaves (or any 1. On a large plate, spread hummus on the bottom. other basil) 2. Mix fruits and herbs, plate in the middle. INTENTION ½ cup fresh lemon juice salt 3. Finish with a nice extra virgin olive oil and some more sumac. This recipe describes the beauty of food evolution. When Israel was born, sumac 4. Serve with warm pita bread. many Jews migrated from different parts of the world, bringing with them extra virgin olive oil ingredients and different culinary traditions.