Ammonium Formate; Exemption from Exemption from the Requirement of a OPP–2006–0121
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

UNITED STATES PATENT OFFICE 2,629,746 PROCESS of PRODUCING PENTAERYTHRTOL Richard F
Patented Feb. 24, 1953 r - 2,629,746 UNITED STATES PATENT OFFICE 2,629,746 PROCESS OF PRODUCING PENTAERYTHRTOL Richard F. B. Cox, Wilmington, Del, assignor to Hercules Powder Company, Wilmington, Del, a corporation of Delaware No Drawing. Application June 12, 1948, Serial No. 32,737 i Caim. (C. 260-635) 2 ihis application is a continuation-in-part of The process to which this invention relates my copending application Serial Number 459,709, may broadly be described as involving condensa filed September 25, 1942, and now abandoned. tion of acetaldehyde with formaldehyde in an This invention relates to an improved process aqueous medium in the presence of an alkaline for the preparation of pentaerythritol. More catalyst to form a reaction mixture comprising particularly, it is concerned with an improved pentaerythritol and the formate of the catalyst process for recovering pentaerythritol from a cation, Converting said reaction mixture to an Crude reaction mixture obtained by the alkaline aqueous Solution comprising pentaerythritol and Condensation of acetaldehyde and formaldehyde. free formic acid by addition of a substance yield Pentaerythritol is prepared commercially by O ing hydrogen ions in an amount at least chemi condensation of acetaldehyde with formaldehyde cally equivalent to the formate present, extract in an aqueous medium in the presence of an ing formic acid from Said solution with a formic alkaline catalyst such as Ca (CH)2, NaOH, etc. acid solvent substantially water-immiscible and in the resulting reaction mixture there is pres nonsolvent for the pentaerythritol, applying heat ent in addition to the pentaerythritol a metal 15 to evaporate said extracted solution at least to Salt of formic acid, the particular salt depend a point at which Crystallization takes place, and ing upon the catalyst enployed. -

Methyl Formate
SIGMA-ALDRICH sigma-aldrich.com Material Safety Data Sheet Version 5.0 Revision Date 09/17/2012 Print Date 03/13/2014 1. PRODUCT AND COMPANY IDENTIFICATION Product name : Methyl formate Product Number : 291056 Brand : Sigma-Aldrich Supplier : Sigma-Aldrich 3050 Spruce Street SAINT LOUIS MO 63103 USA Telephone : +1 800-325-5832 Fax : +1 800-325-5052 Emergency Phone # (For : (314) 776-6555 both supplier and manufacturer) Preparation Information : Sigma-Aldrich Corporation Product Safety - Americas Region 1-800-521-8956 2. HAZARDS IDENTIFICATION Emergency Overview OSHA Hazards Flammable liquid, Target Organ Effect, Harmful by ingestion., Irritant Target Organs Eyes, Kidney GHS Classification Flammable liquids (Category 1) Acute toxicity, Oral (Category 4) Acute toxicity, Inhalation (Category 4) Eye irritation (Category 2A) Specific target organ toxicity - single exposure (Category 3) GHS Label elements, including precautionary statements Pictogram Signal word Danger Hazard statement(s) H224 Extremely flammable liquid and vapour. H302 + H332 Harmful if swallowed or if inhaled H319 Causes serious eye irritation. H335 May cause respiratory irritation. Precautionary statement(s) P210 Keep away from heat/sparks/open flames/hot surfaces. - No smoking. P261 Avoid breathing dust/ fume/ gas/ mist/ vapours/ spray. P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. HMIS Classification Health hazard: 2 Sigma-Aldrich - 291056 Page 1 of 7 Chronic Health Hazard: * Flammability: 4 Physical hazards: 0 NFPA Rating Health hazard: 2 Fire: 4 Reactivity Hazard: 0 Potential Health Effects Inhalation May be harmful if inhaled. Causes respiratory tract irritation. Skin Harmful if absorbed through skin. -

Ammonium Formate As Green Hydrogen Source for Clean Semi-Continuous Enzymatic Dynamic Kinetic Resolution of (+/-)-Ααα-Methylbenzylamine
RSC Advances Ammonium Formate as Green Hydrogen Source for Clean Semi-Continuous Enzymatic Dynamic Kinetic Resolution of (+/-)-ααα-Methylbenzylamine Journal: RSC Advances Manuscript ID: RA-ART-01-2014-000462.R1 Article Type: Paper Date Submitted by the Author: 21-Feb-2014 Complete List of Authors: Miranda, Leandro S. M.; Federal University of Rio de Janeiro, Biocatalysis and Organic Synthesis Lab, Chemistry Institute de Souza, Rodrigo Octavio; Federal University of Rio de Janeiro, de Miranda, Amanda; Federal University of Rio de Janeiro, Page 1 of 21 RSC Advances Graphical Abstract RSC Advances Page 2 of 21 Ammonium Formate as Green Hydrogen Source for Clean Semi-Continuous Enzymatic Dynamic Kinetic Resolution of (+/-)-α- Methylbenzylamine Amanda S. de Miranda, [a] Rodrigo O. M. A. de Souza, [ a] Leandro S. M. Miranda [a]* Keywords: Dynamic kinetic resolution • racemic amines • continuous flow . ammonium formate. Abstract: Abstract: The chemoenzymatic dynamic kinetic resolution of (+/-)-α- Methylbenzylamine under continuous flow conditions in the presence of Pd/BaSO 4 as racemization catalyst and ammonium formate as reductant is described. Under the conditions developed good conversions and excellent enantiomeric excess are reported Page 3 of 21 RSC Advances Introduction Recently, continuous processing and biocatalysis have been elected as key green engineering research areas for sustainable manufacturing 1a and it is clear that joint efforts between these areas can lead to great improvements on continuous manufacturing in agreement with green chemistry principles 1b,c . Optically pure amines are ubiquitously present in nature and active pharmaceutical ingredients (APIs). However, their synthesis still represents an ongoing synthetic challenge that can be inferred by the great amount of work and methodologies dealing with this issue in the literature. -

Mechanochemical Catalytic Transfer Hydrogenation of Aromatic Nitro Derivatives
Article Mechanochemical Catalytic Transfer Hydrogenation of Aromatic Nitro Derivatives Tomislav Portada, Davor Margetić and Vjekoslav Štrukil * Division of Organic Chemistry and Biochemistry, Ruđer Bošković Institute, Bijenička cesta 54, 10000 Zagreb, Croatia; [email protected] (T.P.); [email protected] (D.M.) * Correspondence: [email protected]; Tel.: +385‐1‐468‐0197 Received: 15 November 2018; Accepted: 29 November 2018; Published: date Abstract: Mechanochemical ball milling catalytic transfer hydrogenation (CTH) of aromatic nitro compounds using readily available and cheap ammonium formate as the hydrogen source is demonstrated as a simple, facile and clean approach for the synthesis of substituted anilines and selected pharmaceutically relevant compounds. The scope of mechanochemical CTH is broad, as the reduction conditions tolerate various functionalities, for example nitro, amino, hydroxy, carbonyl, amide, urea, amino acid and heterocyclic. The presented methodology was also successfully integrated with other types of chemical reactions previously carried out mechanochemically, such as amide bond formation by coupling amines with acyl chlorides or anhydrides and click‐type coupling reactions between amines and iso(thio)cyanates. In this way, we showed that active pharmaceutical ingredients Procainamide and Paracetamol could be synthesized from the respective nitro‐precursors on milligram and gram scale in excellent isolated yields. Keywords: mechanochemistry; catalytic transfer hydrogenation; aromatic nitro derivatives; ammonium formate; aging; ball milling; synthesis 1. Introduction Catalytic hydrogenation is one of the most significant functional group transformation reactions in organic synthesis and numerous procedures and reagents have been developed for that purpose [1,2]. As such, the hydrogenation reaction plays one of the key roles in many industrially important processes, for example hydrogenation of carbon monoxide to methanol or in food industry for the conversion of unsaturated vegetable oils into saturated triglycerides [3]. -

Deleterious Effects of Formic Acid Without Salt Additives on the HILIC Analysis of Basic Compounds
HPLC TN-1040 Deleterious Effects of Formic Acid without Salt Additives on the HILIC Analysis of Basic Compounds A. Carl Sanchez and Monika Kansal Phenomenex, Inc., Torrance, CA, USA Abstract Formic acid is an often-used mobile phase additive for of weak acids increase. The pKa shifts can be quite significant adjusting pH in reversed phase liquid chromatography (RPLC), in the high organic environment used for HILIC. For example, especially when using mass spectrometric (MS) detection. This weak bases with aqueous pKa less than ~4 typically will not be practice has been carried over to hydrophilic interaction liquid protonated in HILIC mobile phases when 0.1 v/v % formic acid chromatography (HILIC) separations. However, the mechanisms is used. The pKa of the base is decreased in HILIC mobile phase of action and the relative importance of buffer cation and while the pKa of the formic acid is increased. The increased pKa anion are much different in HILIC than RPLC. For this reason of formic acid leads to an increase in mobile phase pH. The buffer selection in HILIC mode requires consideration of buffer, combination of these opposing changes in pKa results in 0.1 v/v analyte and chromatographic sorbent chemical properties to % formic acid being too weak to protonate bases with pKa < ~4. make an appropriate choice. Proper choice of buffer can make Therefore, formic acid can provide acceptable chromatographic the difference between success and failure with HILIC. In this performance for weak bases with aqueous pKa < ~4. However, paper, the behavior of formic acid with and without the addition basic compounds with aqueous pKa greater than ~4 can be of various salts on the HILIC separation of basic analytes is protonated under HILIC conditions with formic acid. -

UC Irvine UC Irvine Electronic Theses and Dissertations
UC Irvine UC Irvine Electronic Theses and Dissertations Title Steps Toward CO2 Reduction to Methanol via Electrochemical Cascade Catalysis Permalink https://escholarship.org/uc/item/4wx6g1vm Author Mercer, Ian Patrick Publication Date 2020 License https://creativecommons.org/licenses/by/4.0/ 4.0 Peer reviewed|Thesis/dissertation eScholarship.org Powered by the California Digital Library University of California UNIVERSITY OF CALIFORNIA, IRVINE Steps Toward CO2 Reduction to Methanol via Electrochemical Cascade Catalysis THESIS submitted in partial satisfaction of the requirements for the degree of MASTER OF SCIENCE in Chemistry by Ian Patrick Mercer Dissertation Committee: Professor Jenny Y. Yang, Chair Professor William J. Evans Professor Andy S. Borovik 2020 © 2020 Ian Patrick Mercer ii Table of Contents Acknowledgements iv Abstract v Chapter 1: Methyl Formate and Formaldehyde Reduction by Metal Hydrides 1 Chapter 2: Synthesis of Group 8 Metal Complexes of LDMA for the Study of Outer Sphere Interactions 25 Conclusion 33 Experimental 34 References 37 iii Acknowledgements I would like to express my sincere appreciation to my committee, especially my chair, Professor Jenny Y. Yang. Her commitment to science, mentoring, and ‘doing the right thing’ will forever inspire me. Financial support was provided by the University of California, Irvine and US Department of Energy, Office of Science, Office of Basic Energy Sciences Awards DE-SC0012150 and DE- 0000243266. iv Abstract of the Thesis Steps Toward CO2 Reduction to Methanol via Electrochemical Cascade Catalysis by Ian Patrick Mercer Master of Science in Chemistry University of California, Irvine, 2020 Professor Jenny Y. Yang, Chair Research on two independent projects is presented: (1) Homogeneous cascade catalysis has been used for the hydrogenation of CO2 to methanol as it allows for rational tuning of catalyst reactivity and lower reaction temperatures compared to heterogeneous catalysis. -
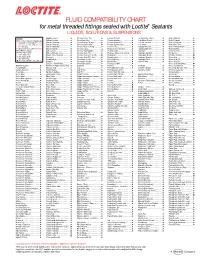
FLUID COMPATIBILITY CHART for Metal Threaded Fittings Sealed with Loctite¨ Sealants LIQUIDS, SOLUTIONS & SUSPENSIONS
FLUID COMPATIBILITY CHART for metal threaded fittings sealed with Loctite® Sealants LIQUIDS, SOLUTIONS & SUSPENSIONS LEGEND: Bagasse Fibers.......................... Chlorobenzene Dry ................... Ferrous Chloride ...................... Ion Exclusion Glycol ................. Nickel Chloride.......................... All Loctite® Anaerobic Sealants are Barium Acetate ........................ Chloroform Dry......................... Ferrous Oxalate......................... Irish Moss Slurry...................... Nickel Cyanide ......................... Compatible Including #242®, 243, Barium Carbonate..................... Chloroformate Methyl............... Ferrous Sulfate10%.................. Iron Ore Taconite ..................... Nickel Fluoborate ..................... 542, 545, 565, 567, 569, 571, 572, Barium Chloride........................ Chlorosulfonic Acid .................. Ferrous Sulfate (Sat)................. Iron Oxide ................................ Nickel Ore Fines ....................... 577, 580, 592 Barium Hydroxide..................... Chrome Acid Cleaning .............. Fertilizer Sol ............................. Isobutyl Alcohol ....................... Nickel Plating Bright ................. † Use Loctite® #270, 271™, 277, 554 Barium Sulfate.......................... Chrome Liquor.......................... Flotation Concentrates.............. Isobutyraldehyde ..................... Nickel Sulfate ........................... Not Recommended Battery Acid .............................. Chrome Plating -

Process for Producing Methyl Methacrylate Verfahren Zur Herstellung Von Methylmethacrylat Procede Pour La Fabrication De Methacrylate De Methyle
~™ II 1 1 III II II 1 1 II II I Ml II I II I II (19) J European Patent Office Office europeen des brevets (11) EP 0 406 676 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publicationation and mention (51 ) |nt. CI.6: C07C 69/54, C07C 67/20 of the grant of the patent: 27.03.1996 Bulletin 1996/13 (21) Application number: 90112194.7 (22) Date of filing: 27.06.1990 (54) Process for producing methyl methacrylate Verfahren zur Herstellung von Methylmethacrylat Procede pour la fabrication de methacrylate de methyle (84) Designated Contracting States: • Ebata, Shuji, DE ES FR GB IT NL C/o Mitsubishi Gas Chem. Com. Tayuhama, Niigata-shi, Niigata-ken (JP) (30) Priority: 04.07.1989 JP 171190/89 (74) Representative: Turk, Gille, Hrabal, Leifert (43) Date of publication of application: Brucknerstrasse 20 09.01.1991 Bulletin 1991/02 D-40593 Dusseldorf (DE) (73) Proprietor: MITSUBISHI GAS CHEMICAL (56) References cited: COMPANY, INC. DE-A- 3 436 608 Chiyoda-ku, Tokyo (JP) • PATENT ABSTRACTS OF JAPAN vol. 14, no. 68 (72) Inventors: (C- 686)(401 1 ), 8 February 1 990; & JP-A-1 290653 • Higuchi, Hirofumi, (MITSUBISHI GAS CHEM) 22.11. 1989 C/o Mitsubishi Gas Chem. Com. Tayuhama, Niigata-shi, Niigata-ken (JP) Remarks: • Kida, Koichi, The file contains technical information submitted C/o Mitsubishi Gas Chem. Com. after the application was filed and not included in this Tayuhama, Niigata-shi, Niigata-ken (JP) specification CO CO CO o Note: Within nine months from the publication of the mention of the grant of the European patent, any person may give notice to the European Patent Office of opposition to the European patent granted. -

Process of Formic Acid Production by Hydrolysis
(19) TZZ ¥_T (11) EP 2 747 883 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: B01J 14/00 (2006.01) C07C 51/09 (2006.01) 15.02.2017 Bulletin 2017/07 C07C 53/02 (2006.01) B01J 10/00 (2006.01) B01D 3/10 (2006.01) C07C 51/44 (2006.01) (21) Application number: 12751504.7 (86) International application number: (22) Date of filing: 27.08.2012 PCT/EP2012/066622 (87) International publication number: WO 2013/030162 (07.03.2013 Gazette 2013/10) (54) PROCESS OF FORMIC ACID PRODUCTION BY HYDROLYSIS OF METHYL FORMATE VERFAHREN ZUR HERSTELLUNG VON AMEISENSÄURE DURCH HYDROLYSE VON METHYLFORMIAT PROCÉDÉ DE PRODUCTION D’ACIDE FORMIQUE PAR HYDROLYSE DE FORMIATE DE MÉTHYLE (84) Designated Contracting States: • TREJBAL, Jiri AL AT BE BG CH CY CZ DE DK EE ES FI FR GB 278 01 Kralupy nad Vltavou (CZ) GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO • ROOSE, Peter PL PT RO RS SE SI SK SM TR 9831 Sint-Martens-Latem (BE) (30) Priority: 27.08.2011 US 201161528204 P (74) Representative: Gevers Patents Intellectual Property House (43) Date of publication of application: Holidaystraat 5 02.07.2014 Bulletin 2014/27 1831 Diegem (BE) (73) Proprietor: Taminco (56) References cited: 9000 Gent (BE) WO-A1-00/39067 US-A- 4 262 140 US-B1- 6 713 649 US-B2- 6 696 603 (72) Inventors: • PASEK, Josef 160 00 Praha 6 (CZ) Note: Within nine months of the publication of the mention of the grant of the European patent in the European Patent Bulletin, any person may give notice to the European Patent Office of opposition to that patent, in accordance with the Implementing Regulations. -

Butyraldehyde Casrn: 123-72-8 Unii: H21352682a
BUTYRALDEHYDE CASRN: 123-72-8 UNII: H21352682A FULL RECORD DISPLAY Displays all fields in the record. For other data, click on the Table of Contents Human Health Effects: Human Toxicity Excerpts: /HUMAN EXPOSURE STUDIES/ Three Asian subjects who reported experiencing severe facial flushing in response to ethanol ingestion were subjects of patch testing to aliphatic alcohols and aldehydes. An aqueous suspension of 75% (v/v) of each alcohol and aldehyde was prepared and 25 uL was used to saturate ashless grade filter paper squares which were then placed on the forearm of each subject. Patches were covered with Parafilm and left in place for 5 minutes when the patches were removed and the area gently blotted. Sites showing erythema during the next 60 minutes were considered positive. All three subjects displayed positive responses to ethyl, propyl, butyl, and pentyl alcohols. Intense positive reactions, with variable amounts of edema, were observed for all the aldehydes tested (valeraldehyde as well as acetaldehyde, propionaldehyde, and butyraldehyde). [United Nations Environment Programme: Screening Information Data Sheets on n-Valeraldehyde (110-62-3) (October 2005) Available from, as of January 15, 2009: http://www.chem.unep.ch/irptc/sids/OECDSIDS/sidspub.html] **PEER REVIEWED** [United Nations Environment Programme: Screening Information Data Sheets on nValeraldehyde (110623) (October 2005) Available from, as of January 15, 2009: http://www.chem.unep.ch/irptc/sids/OECDSIDS/sidspub.html] **PEER REVIEWED** /SIGNS AND SYMPTOMS/ May act as irritant, /SRP: CNS depressant/ ...[Budavari, S. (ed.). The Merck Index - Encyclopedia of Chemicals, Drugs and Biologicals. Rahway, NJ: Merck and Co., Inc., 1989., p. -

Hydrogenation of 2-Methylnaphthalene in a Trickle Bed Reactor Over Bifunctional Nickel Catalysts
The University of Maine DigitalCommons@UMaine Electronic Theses and Dissertations Fogler Library Fall 12-2020 Hydrogenation of 2-methylnaphthalene in a Trickle Bed Reactor Over Bifunctional Nickel Catalysts Matthew J. Kline University of Maine, [email protected] Follow this and additional works at: https://digitalcommons.library.umaine.edu/etd Part of the Catalysis and Reaction Engineering Commons, and the Petroleum Engineering Commons Recommended Citation Kline, Matthew J., "Hydrogenation of 2-methylnaphthalene in a Trickle Bed Reactor Over Bifunctional Nickel Catalysts" (2020). Electronic Theses and Dissertations. 3284. https://digitalcommons.library.umaine.edu/etd/3284 This Open-Access Thesis is brought to you for free and open access by DigitalCommons@UMaine. It has been accepted for inclusion in Electronic Theses and Dissertations by an authorized administrator of DigitalCommons@UMaine. For more information, please contact [email protected]. HYDROGENATION OF 2-METHYLNAPHTHALENE IN A TRICKLE BED REACTOR OVER BIFUNCTIONAL NICKEL CATALYSTS By Matthew J. Kline B.S. Seton Hill University, 2018 A THESIS Submitted in Partial Fulfillment of the Requirements For the Degree of Master oF Science (in Chemical Engineering) The Graduate School The University of Maine December 2020 Advisory Committee: M. Clayton Wheeler, Professor of Chemical Engineering, Advisor Thomas J. Schwartz, Assistant ProFessor oF Chemical Engineering William J. DeSisto, ProFessor oF Chemical Engineering Brian G. Frederick, ProFessor oF Chemistry -

Selected Analytical Methods for Environmental Remediation and Recovery (SAM) 2017
EPA/600/R-17/356 | September 2017 www.epa.gov/homeland-security-research Selected Analytical Methods for Environmental Remediation and Recovery (SAM) 2017 Office of Research and Development Homeland Security Research Program This page left intentionally blank EPA/600/R-17/356 | September 2017 Selected Analytical Methods for Environmental Remediation and Recovery (SAM) 2017 UNITED STATES ENVIRONMENTAL PROTECTION AGENCY Cincinnati, OH 45268 Office of Research and Development Homeland Security Research Program Disclaimer Disclaimer The U.S. Environmental Protection Agency (EPA) through its Office of Research and Development funded and managed the research described here under Contract EP-C-15-012 to CSRA Inc. This document is undergoing review and has not been approved for publication. The contents reflect the views of the contributors and technical work groups and do not necessarily reflect the views of the Agency. Mention of trade names or commercial products in this document or in the methods referenced in this document does not constitute endorsement or recommendation for use. Questions concerning this document or its application should be addressed to: Romy Campisano National Homeland Security Research Center Office of Research and Development (NG16) U.S. Environmental Protection Agency 26 West Martin Luther King Drive Cincinnati, OH 45268 (513) 569-7016 [email protected] Kathy Hall National Homeland Security Research Center Office of Research and Development (NG16) U.S. Environmental Protection Agency 26 West Martin Luther King