(Zygophyllaceae): Complexities in Conservation of Endanger
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pre-Antibiotic Therapy of Syphilis Charles T
University of Kentucky UKnowledge Microbiology, Immunology, and Molecular Microbiology, Immunology, and Molecular Genetics Faculty Publications Genetics 2016 Pre-Antibiotic Therapy of Syphilis Charles T. Ambrose University of Kentucky, [email protected] Right click to open a feedback form in a new tab to let us know how this document benefits oy u. Follow this and additional works at: https://uknowledge.uky.edu/microbio_facpub Part of the Medical Immunology Commons Repository Citation Ambrose, Charles T., "Pre-Antibiotic Therapy of Syphilis" (2016). Microbiology, Immunology, and Molecular Genetics Faculty Publications. 83. https://uknowledge.uky.edu/microbio_facpub/83 This Article is brought to you for free and open access by the Microbiology, Immunology, and Molecular Genetics at UKnowledge. It has been accepted for inclusion in Microbiology, Immunology, and Molecular Genetics Faculty Publications by an authorized administrator of UKnowledge. For more information, please contact [email protected]. Pre-Antibiotic Therapy of Syphilis Notes/Citation Information Published in NESSA Journal of Infectious Diseases and Immunology, v. 1, issue 1, p. 1-20. © 2016 C.T. Ambrose This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. This article is available at UKnowledge: https://uknowledge.uky.edu/microbio_facpub/83 Journal of Infectious Diseases and Immunology Volume 1| Issue 1 Review Article Open Access PRE-ANTIBIOTICTHERAPY OF SYPHILIS C.T. Ambrose, M.D1* 1Department of Microbiology, College of Medicine, University of Kentucky *Corresponding author: C.T. Ambrose, M.D, College of Medicine, University of Kentucky Department of Microbiology, E-mail: [email protected] Citation: C.T. -

Outline of Angiosperm Phylogeny
Outline of angiosperm phylogeny: orders, families, and representative genera with emphasis on Oregon native plants Priscilla Spears December 2013 The following listing gives an introduction to the phylogenetic classification of the flowering plants that has emerged in recent decades, and which is based on nucleic acid sequences as well as morphological and developmental data. This listing emphasizes temperate families of the Northern Hemisphere and is meant as an overview with examples of Oregon native plants. It includes many exotic genera that are grown in Oregon as ornamentals plus other plants of interest worldwide. The genera that are Oregon natives are printed in a blue font. Genera that are exotics are shown in black, however genera in blue may also contain non-native species. Names separated by a slash are alternatives or else the nomenclature is in flux. When several genera have the same common name, the names are separated by commas. The order of the family names is from the linear listing of families in the APG III report. For further information, see the references on the last page. Basal Angiosperms (ANITA grade) Amborellales Amborellaceae, sole family, the earliest branch of flowering plants, a shrub native to New Caledonia – Amborella Nymphaeales Hydatellaceae – aquatics from Australasia, previously classified as a grass Cabombaceae (water shield – Brasenia, fanwort – Cabomba) Nymphaeaceae (water lilies – Nymphaea; pond lilies – Nuphar) Austrobaileyales Schisandraceae (wild sarsaparilla, star vine – Schisandra; Japanese -
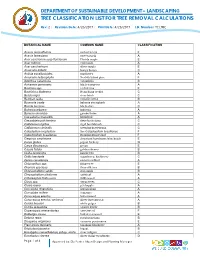
Tree Classification List for Tree Removal Calculations
DEPARTMENT OF SUSTAINABLE DEVELOPMENT – LANDSCAPING TREE CLASSIFICATION LIST FOR TREE REMOVAL CALCULATIONS Rev: 2 | Revision Date: 4/26/2017 | Print Date: 4/26/2017 I.D. Number: TCLTRC BOTANICAL NAME COMMON NAME CLASSIFICATION Acacia auriculiformis earleaf acacia F Acacia farnesiana sweet acacia A Acer saccharum susp floridanum Florida maple E Acer rubrum red maple A Acer saccharinum silver maple E Araucaria bidwilli bunya bunya C Ardisia escallonioides marlberry A Araucaria heterophylla Norfolk Island pine F Averrhoa carambola carambola B Avicennia germinans black mangrove A Bauhinia spp. orchid tree E Bauhinia x blakeana Hong Kong orchid C Betula nigra river birch C Bombax ceiba red silk cotton B Bourreria ovata bahama strongbark A Bucida buceras black olive C Bulnesia arborea bulnesia A Bursera simaruba gumbo limbo A Caesalpinia granadillo bridalveil A Caesalpinia pulcherrima dwarf poinciana C Callistemon rigidus rigid bottlebrush C Callistemon viminalis weeping bottlebrush C Calophyllum inophyllum See Calophyullum brasiliense F Calophyullum brasiliense Brazilian Beautyleaf F Carpinus caroliniana American hornbeam; blue beech E Carya glabra pignut hickory D Carya illinoinensis pecan E Cassia fistula golden shower B Ceiba pentandra kapok tree B Celtis laevigata sugarberry; hackberry C Cercis canadensis eastern redbud A Chionanthus spp. fringe tree E Chorisia speciosa floss-silk tree B Chrysophyllum cainito star-apple B Chrysophyllum oliviforme satinleaf A Citharexylum fruticosum fiddlewood A Citrus spp. citrus trees C Clusia rosea pitchapple A Coccoloba diversifolia pigeon plum A Coccoloba uvifera seagrape A Conocarpus erectus buttonwood A Conocarpus erectus ‘sericeus’ silver buttonwood A Cordia bossieri white geiger B Cordia sebestena orange geiger B Cupaniopsis anacardiodes carrotwood F Dalbergia sissoo Indian rosewood E Delonix regia royal poinciana B Diospyros virginiana common persimmon E Eleocarpus decipens Japanese blueberry A Eriobotrya japonica loquat B Eucalyptus camaldulensis subsp. -

December 2012 Number 1
Calochortiana December 2012 Number 1 December 2012 Number 1 CONTENTS Proceedings of the Fifth South- western Rare and Endangered Plant Conference Calochortiana, a new publication of the Utah Native Plant Society . 3 The Fifth Southwestern Rare and En- dangered Plant Conference, Salt Lake City, Utah, March 2009 . 3 Abstracts of presentations and posters not submitted for the proceedings . 4 Southwestern cienegas: Rare habitats for endangered wetland plants. Robert Sivinski . 17 A new look at ranking plant rarity for conservation purposes, with an em- phasis on the flora of the American Southwest. John R. Spence . 25 The contribution of Cedar Breaks Na- tional Monument to the conservation of vascular plant diversity in Utah. Walter Fertig and Douglas N. Rey- nolds . 35 Studying the seed bank dynamics of rare plants. Susan Meyer . 46 East meets west: Rare desert Alliums in Arizona. John L. Anderson . 56 Calochortus nuttallii (Sego lily), Spatial patterns of endemic plant spe- state flower of Utah. By Kaye cies of the Colorado Plateau. Crystal Thorne. Krause . 63 Continued on page 2 Copyright 2012 Utah Native Plant Society. All Rights Reserved. Utah Native Plant Society Utah Native Plant Society, PO Box 520041, Salt Lake Copyright 2012 Utah Native Plant Society. All Rights City, Utah, 84152-0041. www.unps.org Reserved. Calochortiana is a publication of the Utah Native Plant Society, a 501(c)(3) not-for-profit organi- Editor: Walter Fertig ([email protected]), zation dedicated to conserving and promoting steward- Editorial Committee: Walter Fertig, Mindy Wheeler, ship of our native plants. Leila Shultz, and Susan Meyer CONTENTS, continued Biogeography of rare plants of the Ash Meadows National Wildlife Refuge, Nevada. -

Ehretia Anacua / Condalia Hookeri Forest Texas Ebony – Anacua / Brasíl Forest (From International Vegetation Classification, Natureserve 2012)
6 Major Physiographic Zones of the Lower Rio Grande Valley, Texas (from Hathcock et al. 2014, in press) South Texas Refuge Complex STRC MISSION To restore, enhance, and protect the natural diversity of the Lower Rio Grande Valley of Texas Two-Pronged Approach Acquisition -- land/easements • Create corridors* • Conserve unique biota • Very high, immediate priority Restoration -- mature riparian woodlands • Create corridors* • Augment and enhance habitat blocks • Long-term ecosystem sustainability STRC Restoration Program • Facilitate succession • 5,000 ha planted since mid-1980’s • Early sites direct-seeded/low-density (<600 plants/ha) transplants • Currently 200 ha/year @ 1,000-2,000 plants/ha (50-60 species) • Additional 3,000 ha slated for future Seedlings in “Mini” (6” x 1.5”) Plant Bands Texas ebony Ebanopsis ebano all-thorn goat-bush Castela erecta Evaluation of Effectiveness Traditional • Focus on maximum area/numbers of plants • 1st-Year Survivorship (re-plant?) • No long-term data Current • Increased focus on similarity to natural climax communities • Poor results observed anecdotally at many past sites • Possible to evaluate 15 to 25-year-old sites Study Methods • Non-Systematic, Qualitative Surveys – 2 distinct association-level mature woodland communities – noted dominant species within 4 vertical strata • Belt-Transect Surveys – 9 Sites (3 direct-seed, 5 transplant, 1 control) – counted all individual woody plants within 2 to 3-m belt Ebenopsis ebano – Ehretia anacua / Condalia hookeri Forest Texas Ebony – Anacua / Brasíl -

Research Advances on Leaf and Wood Anatomy of Woody Species
rch: O ea pe es n A R t c s c Rodriguez et al., Forest Res 2016, 5:3 e e r s o s Forest Research F DOI: 10.4172/2168-9776.1000183 Open Access ISSN: 2168-9776 Research Article Open Access Research Advances on Leaf and Wood Anatomy of Woody Species of a Tamaulipan Thorn Scrub Forest and its Significance in Taxonomy and Drought Resistance Rodriguez HG1*, Maiti R1 and Kumari A2 1Universidad Autónoma de Nuevo León, Facultad de Ciencias Forestales, Carr. Nac. No. 85 Km. 45, Linares, Nuevo León 67700, México 2Plant Physiology, Agricultural College, Professor Jaya Shankar Telangana State Agricultural University, Polasa, Jagtial, Karimnagar, Telangana, India Abstract The present paper make a synthesis of a comparative leaf anatomy including leaf surface, leaf lamina, petiole and venation as well as wood anatomy of 30 woody species of a Tamaulipan Thorn Scrub, Northeastern Mexico. The results showed a large variability in anatomical traits of both leaf and wood anatomy. The variations of these anatomical traits could be effectively used in taxonomic delimitation of the species and adaptation of the species to xeric environments. For example the absence or low frequency of stomata on leaf surface, the presence of long palisade cells, and presence of narrow xylem vessels in the wood could be related to adaptation of the species to drought. Besides the species with dense venation and petiole with thick collenchyma and sclerenchyma and large vascular bundle could be well adapted to xeric environments. It is suggested that a comprehensive consideration of leaf anatomy (leaf surface, lamina, petiole and venation) and wood anatomy should be used as a basis of taxonomy and drought resistance. -

Nuclear DNA Content and Chromosome Number of Krameria Cistoidea Hook
Gayana Bot. 74(1): 233-235, 2017. ISSN 0016-5301 Short communication Nuclear DNA content and chromosome number of Krameria cistoidea Hook. & Arn. (Krameriaceae) Contenido de ADN nuclear y número cromosómico de Krameria cistoidea Hook. & Arn. (Krameriaceae) CLAUDIO PALMA-ROJAS1*, PEDRO JARA-SEGUEL2, MARJORIE GARCÍA1 & ELISABETH VON BRAND3 1Departamento de Biología, Facultad de Ciencias, Universidad de La Serena, Casilla 599, La Serena, Chile. 2Escuela de Ciencias Ambientales y Núcleo de Estudios Ambientales, Facultad de Recursos Naturales, Universidad Católica de Temuco, Casilla 15-D, Temuco, Chile. 3Departamento de Biología Marina, Facultad de Ciencias del Mar, Universidad Católica del Norte, Casilla 117, Coquimbo, Chile. *[email protected] RESUMEN Krameria cistoidea Hook. & Arn. tiene un valor 2C de 18,64 + 1,09 pg con un coeficiente de variación de 5,8%. El número cromosómico 2n = 12 descrito para otras seis especies de Krameria está también presente en K. cistoidea. Estos datos citológicos de K. cistoidea son discutidos en relación a antecedentes disponibles para otras Angiospermas, así como para tres géneros de la familia taxonómicamente relacionada Zygophyllaceae. Krameria cistoidea Hook. & Arn. (Krameriaceae) is a the related family Zygophyllaceae (e.g. Larrea, Bulnesia, species endemic to Chile that inhabits coastal and pre- Tribulus). These genera show cytological characters that andean slopes (Squeo et al. 2001), with a center of differ to Krameria species described so far, with diploid distribution located between the rivers Huasco (28ºS) and species (2n = 26), tetraploids (2n = 52), hexaploids (2n = Limari (30ºS) in the semiarid zone. Along its geographical 78) and octoploids (2n = 104), and all having low 2C-values range K. -

Guaiacum Sanctum: Lignum Vitae1 Edward F
ENH445 Guaiacum sanctum: Lignum Vitae1 Edward F. Gilman, Dennis G. Watson, Ryan W. Klein, Andrew K. Koeser, Deborah R. Hilbert, and Drew C. McLean2 Introduction Uses: tree lawn 3–4 feet wide; tree lawn 4–6 feet wide; tree lawn > 6 ft wide; sidewalk cutout (tree pit); parking lot Lignum vitae is an extremely slow-growing broadleaf island < 100 sq ft; parking lot island 100–200 sq ft; parking evergreen which ultimately reaches 30 feet in height and lot island > 200 sq ft; container or planter; specimen; deck casts light shade, but few people have seen plants of this or patio; Bonsai; highway median size because it is not grown in the trade. Most are seen 8 to 12 feet tall with a beautiful array of multiple trunks and a rounded canopy much like that of a mature crape-myrtle. The one to two-inch-long, leathery, dark green leaves are joined at many times throughout the year by the production of large clusters of bluish purple flowers, the old flowers fading to a light silvery-blue and creating a shimmering haze over the rounded canopy. These flowers are followed by small, heart-shaped, yellow orange berries, appearing on the tree at the same time as the bluish purple flowers and creating a lovely sight. General Information Figure 1. Full Form—Guaiacum sanctum: Lignum vitae Scientific name: Guaiacum sanctum Description Pronunciation: GWY-uh-kum SANK-tum Height: 10 to 30 feet Common name(s): Lignum vitae, holywood, tree of life Spread: 8 to 12 feet Family: Zygophyllaceae Crown uniformity: symmetrical USDA hardiness zones: 10B through 11 (Figure 2) Crown shape: round, vase Origin: native to Florida, the West Indies, Mexico, and Crown density: dense Central America Growth rate: slow UF/IFAS Invasive Assessment Status: native Texture: fine 1. -

Morfoanatomía Floral De Kallstroemia Maxima (Zygophyllaceae)
Revista Mexicana de Biodiversidad Revista Mexicana de Biodiversidad 90 (2019): e902075 Anatomía Morfoanatomía floral de Kallstroemia maxima (Zygophyllaceae) Floral morphoanatomy of Kallstroemia maxima (Zygophyllaceae) Rosa María Fonseca a, *, Mercedes Eunice Castro-Laportte b y Estela Sandoval-Zapotitla c a Facultad de Ciencias, Universidad Nacional Autónoma de México, Circuito Exterior s/n, Ciudad Universitaria, 04510 Ciudad de México, México b Facultad de Agronomía, Universidad Central de Venezuela, Maracay 2101, Aragua, Venezuela c Jardín Botánico, Instituto de Biología, Universidad Nacional Autónoma de México, Tercer Circuito Exterior s/n, Ciudad Universitaria, 04510 Ciudad de México, México *Autor para correspondencia: [email protected] (R.M. Fonseca) Recibido: 9 enero 2016; aceptado: 30 julio 2017 Resumen El objetivo es contribuir al conocimiento de la anatomía floral de Kallstroemia maxima y compararlo con otros géneros relacionados dentro de la familia Zygophyllaceae. Se utilizaron botones florales de 3 plantas de K. maxima recolectados el día previo a la antesis; se procesaron preparaciones permanentes y se hicieron observaciones a partir de fotomicrografías de botones herborizados. Se proporcionan descripciones de algunas estructuras anatómicas no descritas con anterioridad, como son: nectarios opuestos a los sépalos, presencia de un haz vascular compartido por un pétalo y el estambre opuesto a éste, engrosamientos helicoidales en las células epidérmicas de los márgenes de los pétalos, células papilares en la superficie abaxial de los pétalos cerca de su base, número de haces vasculares que irrigan a cada carpelo y la existencia de una cavidad interna en la base del estilo. Los resultados permiten distinguir mejor a K. maxima de los géneros afines dentro de las Zygophyllaceae. -

Texas Big Tree Registry a List of the Largest Trees in Texas Sponsored by Texas a & M Forest Service
Texas Big Tree Registry A list of the largest trees in Texas Sponsored by Texas A & M Forest Service Native and Naturalized Species of Texas: 320 ( D indicates species naturalized to Texas) Common Name (also known as) Latin Name Remarks Cir. Threshold acacia, Berlandier (guajillo) Senegalia berlandieri Considered a shrub by B. Simpson 18'' or 1.5 ' acacia, blackbrush Vachellia rigidula Considered a shrub by Simpson 12'' or 1.0 ' acacia, Gregg (catclaw acacia, Gregg catclaw) Senegalia greggii var. greggii Was named A. greggii 55'' or 4.6 ' acacia, Roemer (roundflower catclaw) Senegalia roemeriana 18'' or 1.5 ' acacia, sweet (huisache) Vachellia farnesiana 100'' or 8.3 ' acacia, twisted (huisachillo) Vachellia bravoensis Was named 'A. tortuosa' 9'' or 0.8 ' acacia, Wright (Wright catclaw) Senegalia greggii var. wrightii Was named 'A. wrightii' 70'' or 5.8 ' D ailanthus (tree-of-heaven) Ailanthus altissima 120'' or 10.0 ' alder, hazel Alnus serrulata 18'' or 1.5 ' allthorn (crown-of-thorns) Koeberlinia spinosa Considered a shrub by Simpson 18'' or 1.5 ' anacahuita (anacahuite, Mexican olive) Cordia boissieri 60'' or 5.0 ' anacua (anaqua, knockaway) Ehretia anacua 120'' or 10.0 ' ash, Carolina Fraxinus caroliniana 90'' or 7.5 ' ash, Chihuahuan Fraxinus papillosa 12'' or 1.0 ' ash, fragrant Fraxinus cuspidata 18'' or 1.5 ' ash, green Fraxinus pennsylvanica 120'' or 10.0 ' ash, Gregg (littleleaf ash) Fraxinus greggii 12'' or 1.0 ' ash, Mexican (Berlandier ash) Fraxinus berlandieriana Was named 'F. berlandierana' 120'' or 10.0 ' ash, Texas Fraxinus texensis 60'' or 5.0 ' ash, velvet (Arizona ash) Fraxinus velutina 120'' or 10.0 ' ash, white Fraxinus americana 100'' or 8.3 ' aspen, quaking Populus tremuloides 25'' or 2.1 ' baccharis, eastern (groundseltree) Baccharis halimifolia Considered a shrub by Simpson 12'' or 1.0 ' baldcypress (bald cypress) Taxodium distichum Was named 'T. -

Is Guaiacum Sanctum Effective Against Arthritis? an Ethnobotany
Is Guaiacum sanctum Effective Against Arthritis? An Ethnobotany Case by Eric Ribbens, Barbra Burdett, and Angela Green Department of Biological Science Western Illinois University Part I—Anecdotal Evidence Dr. Beth Tonoany is a tropical population ecologist who has been studying an unusual tree, Guaiacum sanctum, which once grew throughout the dry tropical forests of Central America as well as on some of the Caribbean islands. Guaiacum sanctum produces a wood called lignum vitae, and is known in Costa Rica and other Spanish-speaking countries as guayacan reál. The wood is extremely heavy because it contains extensive deposits of resin (Howes, 1949) and it will sink if placed in water (Wilson and Eisner, 1968). During World War I and II it was extensively harvested for use in the ship-building industry because the wood, which does not split easily, is self- lubricating due to its high resin content. The wood is very durable, and was in high demand for constructing bearing sleeves to support ship propellor Figure 1: Seeds of shafts (Scurlock, 1987). It has also been used for making railroad ties Guaiacum sanctum (Woods, 1951). Dr. Tonoany has been studying one of the last remaining populations of lignum vitae in the Palo Verde Nature Preserve in northwestern Costa Rica. Probably fewer than 100 trees remain in Costa Rica, most in the Palo Verde Nature Preserve. Her research has included tracking seedlings and saplings, locating and measuring adult trees, and interviewing some of the local Ticos to learn about the tree’s past history in Costa Rica. The tree, while rare now due to the dramatic conversion of tropical deciduous forest in Costa Rica into pasturelands and to selective logging of the tree for its valuable wood, was once more common, and many of the older Ticos remember that the saplings were used to make cattle switches because of the strong flexible wood in the saplings. -

South American Cacti in Time and Space: Studies on the Diversification of the Tribe Cereeae, with Particular Focus on Subtribe Trichocereinae (Cactaceae)
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2013 South American Cacti in time and space: studies on the diversification of the tribe Cereeae, with particular focus on subtribe Trichocereinae (Cactaceae) Lendel, Anita Posted at the Zurich Open Repository and Archive, University of Zurich ZORA URL: https://doi.org/10.5167/uzh-93287 Dissertation Published Version Originally published at: Lendel, Anita. South American Cacti in time and space: studies on the diversification of the tribe Cereeae, with particular focus on subtribe Trichocereinae (Cactaceae). 2013, University of Zurich, Faculty of Science. South American Cacti in Time and Space: Studies on the Diversification of the Tribe Cereeae, with Particular Focus on Subtribe Trichocereinae (Cactaceae) _________________________________________________________________________________ Dissertation zur Erlangung der naturwissenschaftlichen Doktorwürde (Dr.sc.nat.) vorgelegt der Mathematisch-naturwissenschaftlichen Fakultät der Universität Zürich von Anita Lendel aus Kroatien Promotionskomitee: Prof. Dr. H. Peter Linder (Vorsitz) PD. Dr. Reto Nyffeler Prof. Dr. Elena Conti Zürich, 2013 Table of Contents Acknowledgments 1 Introduction 3 Chapter 1. Phylogenetics and taxonomy of the tribe Cereeae s.l., with particular focus 15 on the subtribe Trichocereinae (Cactaceae – Cactoideae) Chapter 2. Floral evolution in the South American tribe Cereeae s.l. (Cactaceae: 53 Cactoideae): Pollination syndromes in a comparative phylogenetic context Chapter 3. Contemporaneous and recent radiations of the world’s major succulent 86 plant lineages Chapter 4. Tackling the molecular dating paradox: underestimated pitfalls and best 121 strategies when fossils are scarce Outlook and Future Research 207 Curriculum Vitae 209 Summary 211 Zusammenfassung 213 Acknowledgments I really believe that no one can go through the process of doing a PhD and come out without being changed at a very profound level.