Current Status of Augmentation and Combination Treatments for Major Depressive Disorder: a Literature Review and a Proposal for a Novel Approach to Improve Practice
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pindolol of the Activation of Postsynaptic 5-HT1A Receptors
Potentiation by (-)Pindolol of the Activation of Postsynaptic 5-HT1A Receptors Induced by Venlafaxine Jean-Claude Béïque, Ph.D., Pierre Blier, M.D., Ph.D., Claude de Montigny, M.D., Ph.D., and Guy Debonnel, M.D. The increase of extracellular 5-HT in brain terminal regions antagonist WAY 100635 (100 g/kg, i.v.). A short-term produced by the acute administration of 5-HT reuptake treatment with VLX (20 mg/kg/day ϫ 2 days) resulted in a inhibitors (SSRI’s) is hampered by the activation of ca. 90% suppression of the firing activity of 5-HT neurons somatodendritic 5-HT1A autoreceptors in the raphe nuclei. in the dorsal raphe nucleus. This was prevented by the The present in vivo electrophysiological studies were coadministration of (-)pindolol (15 mg/kg/day ϫ 2 days). undertaken, in the rat, to assess the effects of the Taken together, these results indicate that (-)pindolol coadministration of venlafaxine, a dual 5-HT/NE reuptake potentiated the activation of postsynaptic 5-HT1A receptors inhibitor, and (-)pindolol on pre- and postsynaptic 5-HT1A resulting from 5-HT reuptake inhibition probably by receptor function. The acute administration of venlafaxine blocking the somatodendritic 5-HT1A autoreceptor, but not and of the SSRI paroxetine (5 mg/kg, i.v.) induced a its postsynaptic congener. These results support and extend suppression of the firing activity of dorsal hippocampus CA3 previous findings providing a biological substratum for the pyramidal neurons. This effect of venlafaxine was markedly efficacy of pindolol as an accelerating strategy in major potentiated by a pretreatment with (-)pindolol (15 mg/kg, depression. -
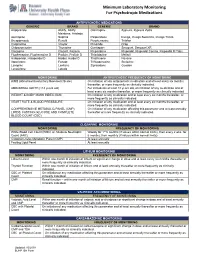
Minimum Laboratory Monitoring for Psychotropic Medications
Minimum Laboratory Monitoring For Psychotropic Medications ANTIPSYCHOTIC MEDICATIONS GENERIC BRAND GENERIC BRAND Aripiprazole Abilify, Abilify Olanzapine Zyprexa, Zyprexa Zydis Maintena, Aristada Asenapine Saphris Paliperidone Invega, Invega Sustenna, Invega Trinza Brexpiprazole Rexulti Perphenazine Trilafon Cariprazine Vraylar Pimozide Orap Chlorpromazine Thorazine Quetiapine Seroquel, Seroquel XR Clozapine Clozaril, Fazaclo Risperidone Risperdal, Risperdal Consta, Risperdal M Tabs Fluphenazine, Fluphenazine D Prolixin, Prolixin D Thioridazine Mellaril Haloperidol, Haloperidol D Haldol, Haldol D Thiothixene Navane Iloperidone Fanapt Trifluoperazine Stelazine Loxapine Loxitane Ziprasidone Geodon Lurasidone Latuda MONITORING ANTIPSYCHOTIC FREQUENCY OF MONITORING AIMS (Abnormal Involuntary Movement Scale) On initiation of any antipsychotic medication and at least every six months thereafter, or more frequently as clinically indicated. ABDOMINAL GIRTH (>18 years old) For individuals at least 18 years old, on initiation of any medication and at least every six months thereafter, or more frequently as clinically indicated. WEIGHT & BODY MASS INDEX (BMI) On initiation of any medication and at least every six months thereafter, or more frequently as clinically indicated. HEART RATE & BLOOD PRESSSURE On initiation of any medication and at least every six months thereafter, or more frequently as clinically indicated. COMPREHENSIVE METABOLIC PANEL (CMP), On initiation of any medication affecting this parameter and at least annually LIPIDS, FASTING -

Schizophrenia Care Guide
August 2015 CCHCS/DHCS Care Guide: Schizophrenia SUMMARY DECISION SUPPORT PATIENT EDUCATION/SELF MANAGEMENT GOALS ALERTS Minimize frequency and severity of psychotic episodes Suicidal ideation or gestures Encourage medication adherence Abnormal movements Manage medication side effects Delusions Monitor as clinically appropriate Neuroleptic Malignant Syndrome Danger to self or others DIAGNOSTIC CRITERIA/EVALUATION (PER DSM V) 1. Rule out delirium or other medical illnesses mimicking schizophrenia (see page 5), medications or drugs of abuse causing psychosis (see page 6), other mental illness causes of psychosis, e.g., Bipolar Mania or Depression, Major Depression, PTSD, borderline personality disorder (see page 4). Ideas in patients (even odd ideas) that we disagree with can be learned and are therefore not necessarily signs of schizophrenia. Schizophrenia is a world-wide phenomenon that can occur in cultures with widely differing ideas. 2. Diagnosis is made based on the following: (Criteria A and B must be met) A. Two of the following symptoms/signs must be present over much of at least one month (unless treated), with a significant impact on social or occupational functioning, over at least a 6-month period of time: Delusions, Hallucinations, Disorganized Speech, Negative symptoms (social withdrawal, poverty of thought, etc.), severely disorganized or catatonic behavior. B. At least one of the symptoms/signs should be Delusions, Hallucinations, or Disorganized Speech. TREATMENT OPTIONS MEDICATIONS Informed consent for psychotropic -

THE USE of MIRTAZAPINE AS a HYPNOTIC O Uso Da Mirtazapina Como Hipnótico Francisca Magalhães Scoralicka, Einstein Francisco Camargosa, Otávio Toledo Nóbregaa
ARTIGO ESPECIAL THE USE OF MIRTAZAPINE AS A HYPNOTIC O uso da mirtazapina como hipnótico Francisca Magalhães Scoralicka, Einstein Francisco Camargosa, Otávio Toledo Nóbregaa Prescription of approved hypnotics for insomnia decreased by more than 50%, whereas of antidepressive agents outstripped that of hypnotics. However, there is little data on their efficacy to treat insomnia, and many of these medications may be associated with known side effects. Antidepressants are associated with various effects on sleep patterns, depending on the intrinsic pharmacological properties of the active agent, such as degree of inhibition of serotonin or noradrenaline reuptake, effects on 5-HT1A and 5-HT2 receptors, action(s) at alpha-adrenoceptors, and/or histamine H1 sites. Mirtazapine is a noradrenergic and specific serotonergic antidepressive agent that acts by antagonizing alpha-2 adrenergic receptors and blocking 5-HT2 and 5-HT3 receptors. It has high affinity for histamine H1 receptors, low affinity for dopaminergic receptors, and lacks anticholinergic activity. In spite of these potential beneficial effects of mirtazapine on sleep, no placebo-controlled randomized clinical trials of ABSTRACT mirtazapine in primary insomniacs have been conducted. Mirtazapine was associated with improvements in sleep on normal sleepers and depressed patients. The most common side effects of mirtazapine, i.e. dry mouth, drowsiness, increased appetite and increased body weight, were mostly mild and transient. Considering its use in elderly people, this paper provides a revision about studies regarding mirtazapine for sleep disorders. KEYWORDS: sleep; antidepressive agents; sleep disorders; treatment� A prescrição de hipnóticos aprovados para insônia diminuiu em mais de 50%, enquanto de antidepressivos ultrapassou a dos primeiros. -

Does Curcumin Or Pindolol Potentiate Fluoxetine's Antidepressant Effect
Research Paper Does Curcumin or Pindolol Potentiate Fluoxetine’s Antidepressant Effect by a Pharmacokinetic or Pharmacodynamic Interaction? H. A. S. MURAD*, M. I. SULIAMAN1, H. ABDALLAH2 AND MAY ABDULSATTAR1 Department of Pharmacology, Faculty of Medicine, Rabigh, King Abdulaziz University (KAU), Jeddah-21589, Saudi Arabia (SA) and Faculty of Medicine, Ain Shams University, Cairo-11541, Egypt. 1Department of Pharmacology, Faculty of Medicine, KAU, Jeddah-21589, SA. 2Department of Natural Products, Faculty of Pharmacy, KAU, Jeddah-21589, SA and Department of Pharmacognosy, Faculty of Pharmacy, Cairo University, Cairo-11541, Egypt Murad, et al.: Potentiation of Fluoxetine by Curcumin This study was designed to study potentiation of fluoxetine’s antidepressant effect by curcumin or pindolol. Twenty eight groups of mice (n=8) were used in three sets of experiments. In the first set, 9 groups were subjected to the forced swimming test after being treated intraperitoneally with three vehicles, fluoxetine (5 and 20 mg/kg), curcumin (20 mg/kg), pindolol (32 mg/kg), curcumin+fluoxetine (5 mg/kg) and pindolol+fluoxetine (5 mg/kg). One hour after the test, serum and brain fluoxetine and norfluoxetine levels were measured in mice receiving fluoxetine (5 and 20 mg/kg), curcumin+fluoxetine (5 mg/kg) and pindolol+fluoxetine (5 mg/kg). In the second set, the test was done after pretreatment with p-chlorophenylalanine. In the third set, the locomotor activity was measured. The immobility duration was significantly decreased in fluoxetine (20 mg/kg), curcumin (20 mg/kg), curcumin+fluoxetine (5 mg/kg) and pindolol+fluoxetine (5 mg/kg) groups. These decreases were reversed with p-chlorophenylalanine. -

Serotonin and Drug-Induced Therapeutic Responses in Major Depression, Obsessive–Compulsive and Panic Disorders Pierre Blier, M.D., Ph.D
Serotonin and Drug-Induced Therapeutic Responses in Major Depression, Obsessive–Compulsive and Panic Disorders Pierre Blier, M.D., Ph.D. and Claude de Montigny, M.D., Ph.D. The therapeutic effectiveness of antidepressant drugs in major development of treatment strategies producing greater efficacy depression was discovered by pure serendipity. It took over 20 and more rapid onset of antidepressant action; that, is lithium years before the neurobiological modifications that could addition and pindolol combination, respectively. It is expected mediate the antidepressive response were put into evidence. that the better understanding recently obtained of the Indeed, whereas the immediate biochemical effects of these mechanism of action of certain antidepressant drugs in drugs had been well documented, their antidepressant action obsessive– compulsive and panic disorders will also lead to generally does not become apparent before 2 to 3 weeks of more effective treatment strategies for those disorders. treatment. The different classes of antidepressant [Neuropsychopharmacology 21:91S–98S, 1999] treatments were subsequently shown to enhance serotonin © 1999 American College of Neuropsychopharmacology. neurotransmission albeit via different pre- and postsynaptic Published by Elsevier Science Inc. mechanisms. Clinical trials based on this hypothesis led to the KEY WORDS: Antidepressant; Electroconvulsive shocks; electroconvulsive shock treatment (ECT), which was Lithium; Pindolol; Hippocampus; Orbito-frontal cortex generally given only to hospitalized patients. It took several years after the demonstration of the In the late 1950s, the therapeutic effect of certain drugs antidepressant effect of iproniazide and imipramine to in major depression was discovered by serendipity. An understand that these drugs could interfere with the ca- antidepressant response was observed in patients with tabolism of monoamines. -

Current P SYCHIATRY
Current p SYCHIATRY N ew Investigators Tips to manage and prevent discontinuation syndromes Informed tapering can protect patients when you stop a medication Sriram Ramaswamy, MD Shruti Malik, MBBS, MHSA Vijay Dewan, MD Instructor, department of psychiatry Foreign medical graduate Assistant professor Creighton University Department of psychiatry Omaha, NE University of Nebraska Medical Center Omaha, NE bruptly stopping common psychotropics New insights on psychotropic A —particularly antidepressants, benzodi- drug safety and side effects azepines, or atypical antipsychotics—can trigger a discontinuation syndrome, with: This paper was among those entered in the 2005 • rebound or relapse of original symptoms Promising New Investigators competition sponsored • uncomfortable new physical and psycho- by the Neuroleptic Malignant Syndrome Information Service (NMSIS). The theme of this year’s competition logical symptoms was “New insights on psychotropic drug safety and • physiologic withdrawal at times. side effects.” To increase health professionals’ awareness of URRENT SYCHIATRY 1 C P is honored to publish this peer- the risk of these adverse effects, this article reviewed, evidence-based article on a clinically describes discontinuation syndromes associated important topic for practicing psychiatrists. with various psychotropics and offers strategies to NMSIS is dedicated to reducing morbidity and anticipate, recognize, and manage them. mortality of NMS by improving medical and psychiatric care of patients with heat-related disorders; providing -

Clinical Review, Adverse Events
Clinical Review, Adverse Events Drug: Carbamazepine NDA: 16-608, Tegretol 20-712, Carbatrol 21-710, Equetro Adverse Event: Stevens-Johnson Syndrome Reviewer: Ronald Farkas, MD, PhD Medical Reviewer, DNP, ODE I 1. Executive Summary 1.1 Background Carbamazepine (CBZ) is an anticonvulsant with FDA-approved indications in epilepsy, bipolar disorder and neuropathic pain. CBZ is associated with Stevens-Johnson syndrome (SJS) and Toxic Epidermal Necrolysis (TEN), closely related serious cutaneous adverse drug reactions that can be permanently disabling or fatal. Other anticonvulsants, including phenytoin, phenobarbital, and lamotrigine are also associated with SJS/TEN, as are members of a variety of other drug classes, including nonsteriodal anti-inflammatory drugs and sulfa drugs. The incidence of CBZ-associated SJS/TEN has been considered “extremely rare,” as noted in current U.S. drug labeling. However, recent publications and postmarketing data suggest that CBZ- associated SJS/TEN occurs at a much higher rate in some Asian populations, about 2.5 cases per 1,000 new exposures, and that most of this increased risk is in individuals carrying a specific human leukocyte antigen (HLA) allele, HLA-B*1502. This HLA-B allele is present in about 5- to 20% of many, but not all, Asian populations, and is also present in about 2- to 4% of South Asians/Indians. The allele is also present at a lower frequency, < 1%, in several other ethnic groups around the world (although likely due to distant Asian ancestry). About 10% of U.S. Asians carry HLA-B*1502. HLA-B*1502 is generally not present in the U.S. -

Repeated Lysergic Acid Diethylamide in an Animal Model of Depression
JOP0010.1177/0269881114531666Journal of PsychopharmacologyBuchborn et al. 531666research-article2014 Original Paper Repeated lysergic acid diethylamide in an animal model of depression: Normalisation of learning behaviour and Journal of Psychopharmacology 2014, Vol. 28(6) 545 –552 hippocampal serotonin 5-HT signalling © The Author(s) 2014 2 Reprints and permissions: sagepub.co.uk/journalsPermissions.nav DOI: 10.1177/0269881114531666 jop.sagepub.com Tobias Buchborn, Helmut Schröder, Volker Höllt and Gisela Grecksch Abstract A re-balance of postsynaptic serotonin (5-HT) receptor signalling, with an increase in 5-HT1A and a decrease in 5-HT2A signalling, is a final common pathway multiple antidepressants share. Given that the 5-HT1A/2A agonist lysergic acid diethylamide (LSD), when repeatedly applied, selectively downregulates 5-HT2A, but not 5-HT1A receptors, one might expect LSD to similarly re-balance the postsynaptic 5-HT signalling. Challenging this idea, we use an animal model of depression specifically responding to repeated antidepressant treatment (olfactory bulbectomy), and test the antidepressant-like properties of repeated LSD treatment (0.13 mg/kg/d, 11 d). In line with former findings, we observe that bulbectomised rats show marked deficits in active avoidance learning. These deficits, similarly as we earlier noted with imipramine, are largely reversed by repeated LSD administration. Additionally, bulbectomised rats exhibit distinct anomalies of monoamine receptor signalling in hippocampus and/or frontal cortex; 35 from these, only the hippocampal decrease in 5-HT2 related [ S]-GTP-gamma-S binding is normalised by LSD. Importantly, the sham-operated rats do not profit from LSD, and exhibit reduced hippocampal 5-HT2 signalling. As behavioural deficits after bulbectomy respond to agents classified as antidepressants only, we conclude that the effect of LSD in this model can be considered antidepressant-like, and discuss it in terms of a re-balance of hippocampal 5-HT2/5-HT1A signalling. -

Antidepressants the Old and the New
Antidepressants The Old and The New October, 1998 iii In 1958 researchers discovered that imipramine had antidepressant 1 activity. Since then, a number of antidepressants have been HIGHLIGHTS developed with a variety of pharmacological mechanisms and side effect profiles. All antidepressants show similar efficacy in the treatment of depression when used in adequate dosages. Choosing the most PHARMACOLOGY & CLASSIFICATION appropriate agent depends on specific patient variables, The mechanism of action for antidepressants is not entirely clear; concurrent diseases, concurrent drugs, and cost. however they are known to interfere with neurotransmitters. Non-TCA antidepressants such as the SSRIs have become Tricyclic antidepressants (TCAs) block the reuptake of both first line agents in the treatment of depression due to their norepinephrine (NE) and serotonin (5HT). The relative ratio of relative safety and tolerability. Each has its own advantages and their effect on NE versus 5HT varies. The potentiation of NE and disadvantages for consideration in individualizing therapy. 5HT results in changes in the neuroreceptors and is thought to be TCAs may be preferred in patients who do not respond to or the primary mechanism responsible for the antidepressant effect. tolerate other antidepressants, have chronic pain or migraine, or In addition to the effects on NE and 5HT, TCAs also block for whom drug cost is a significant factor. muscarinic, alpha1 adrenergic, and histaminic receptors. The extent of these effects vary with each agent resulting in differing Secondary amine TCAs (desipramine and nortriptyline) have side effect profiles. fewer side effects than tertiary amine TCAs. Maintenance therapy at full therapeutic dosages should be Selective serotonin-reuptake inhibitors (SSRIs) block the 2 considered for patients at high risk for recurrence. -

Medication: Trazodone (Desyrel) 50 Mg
Trazodone COMPLEX CHRONIC DISEASES PROGRAM Medication Handout Date: May 15, 2018 Medication: Trazodone 50 mg What is trazodone: Trazodone is an antidepressant that is now used for insomnia; it helps with both falling asleep and staying asleep. Expected Benefit: As a sleep aid, you should notice a benefit on the first night within about 30 minutes of taking the medication. Watch for possible side effects: This list of side effects is important for you to be aware of however, it is also important to remember that not all side effects happen to everyone. If you have problems with these side effects talk with your doctor or pharmacist: Hangover effect (drowsiness that continues after waking up in the morning) Dizziness Dry mouth Headache Nausea Stopping the medication: Stopping trazodone is not usually a problem as there is no withdrawal effect you are taking it regularly at higher doses than prescribed below. Please ask your doctor or pharmacist before stopping the medication. Rebound insomnia is not usually a problem which makes trazodone a good option for taking a sleeping aid as needed How to use this medication: Take this medication with or without food Dosing Schedule: Start with 12.5 mg (¼ tablet) or 25 mg (½ tablet) at bedtime Increase the dose by ¼ or ½ a tablet every night until: o You can fall asleep, and/or o Stay asleep The usual effective dose is 50 – 150 mg at bedtime (1 – 3 tablets) Do not continue increasing the dose if you experience a hangover effect Talk with your doctor if you are still having problems with sleep, as you may need a different medication. -

Trazodone Is Not As Effective As Other Antidepressants in Treating a Patient with Major Depressive Disorder
Case Report Taiwanese Journal of Psychiatry (Taipei) Vol. 26 No. 4 2012 • 311 • Trazodone Is Not as Effective as Other Antidepressants in Treating a Patient with Major Depressive Disorder Kah Kheng Goh, M.D.1, Weng-Kin Tam, M.D.1, Winston W. Shen, M.D.1, 2* Background: Trazodone in clinical use is ineffective because of its sed ation to let patient receive an adequate dose for treating a patient with major depressive disorder. We are report a case report to demonstrate this clinical effi cacy issue in the treatment with trazodone. Case Report: A 83-year old male Taiwanese patient had suffered from MDD for six months. He did not response to the treatment of trazodone 150 mg/day for four months, but consequently, he responded to the treatment of milnacipran 100 mg/day or mirtazapine 30 mg/day. Conclusion: Psy- chiatrists in Taiwan should be alert to the fact that trazodone is not as effective as other antidepressants, and that trazodone should not be prescribed as a single anti- depressant in treating an MDD patient. Key words: antidepressant therapy, suicide, venlafaxine, bupropion (Taiwanese Journal of Psychiatry [Taipei] 2012; 26: 311-5) guideline in the paragraph in describing individu- Introduction al drug trazodone also indicates “other investiga- tors found trazodone to be less effective than other The practice guideline of the American antidepressant medications” [1]. In this case re- Psychiatric Association in the paragraph of intro- port here, we are reporting a case of a major de- ducing antidepressants states “Although some pressive disorder (MDD) patient, who did not re- studies have suggested superiority of one mecha- spond to daily dose of 150 mg of trazodone, but nism of action over another, there are no replica- responded well to antidepressants 100 mg of mil- ble or robust fi ndings to establish a clinically nacipran per day and maybe 30 mg of mirtazapine meaningful difference.