Download Brochure
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

6.1Road Transportation
Preparation of Sub Regional Plan for Haryana Sub-Region of NCR-2021: Interim Report -II Chapter 6 : TRANSPORTATION 6.1 Road Transportation Since the formation of Haryana state, there has been a significant growth in the road transportation sector of Haryana. As on year 2001, around 23, 000 km of roads connect to villages and cities in Haryana state and with its neighbor states. At present more than 99.88 percentages of villages are connected by metalled roads and road density is around 63.8 km per 100 sq. km area. Length of different types of roads in Haryana State is as follows: National Highways : 1,346 km State Highways : 2,559 km Major District Roads : 1,569 km Other Distt. & village roads : 14,730 km Other roads : 2,852 km Source: Statistical Abstract Haryana, 2006-07 However, economic development in the state is taking place at very higher rate in comparison to other states of India. This is the reason for large density of vehicles on these available roads. As per the information available for the year 2003-2004, about 5763 motor vehicles accommodated within 100 square kilometer of area. Though, the registered number of vehicles as on 31st march 2004 were 25, 47,910, in actual about 28, 53,667 number of motor vehicles traveled on roads of Haryana sate. This shows that a significant percentage of through traffic passes through Haryana state. This large volume of traffic may cause road accidents which results in huge loss of economy and human resources, if proper transportation facilities are not provided. -

Piyush Square Studio Apartments
https://www.propertywala.com/piyush-square-studio-apartments-bhiwadi Piyush Square Studio Apartments - Piyush City… Piyush mission is to rekindle "essence of life" Piyush Square(Studio Apartments)is a Premium Residential Project built to create a vibrant place to live with facilities and amenities as that of premium Residential Project. Project ID : J919062461 Builder: Piyush Group Properties: Apartments / Flats, Independent Houses, Residential Plots / Lands Location: Piyush Square Studio Apartments, Piyush City, Bhiwadi (Rajasthan) Completion Date: Mar, 2013 Status: Started Description Piyush Group is a diversified group with a significant presence in real estate development, construction and financial services.It envisages a project in every major city of the National Capital Region ( NCR ). Residential Projects on the anvil include Integrated Townships in Palwal and Bhiwadi and a world class Group Housing Project (Piyush Heights) is under speed development spread over 17.5 acres (approx.) located at Sec 89, Faridabad . Commercial projects including Upcoming Business Park & Global i being designed by the renowned architect Hafeez Contractor located on Mathura Road , Faridabad. Piyush Square Studio Apartments Within An Elegant, Township Project Of 50 Acres. Piyush City, Bhiwadi. Strategically Located In One Of The Largest And Fastest Growing Industrial Town Of North India, Piyush City, Bhiwadi Only A Five Minute Drive From Nh-8 &, Just 30 Minutes Drive From Gurgaon And Only 45 Minute Drive From Igi Airport, Delhi. Amenities Atm Hospital -

Table of Contents
TABLE OF CONTENTS Reference to Paragraphs Page Preface vii Overview ix Chapter – 1 Introduction Budget profile 1.1 1 Application of resources of the State Government 1.2 1 Persistent savings 1.3 2 Funds transferred directly to the State implementing 1.4 2 agencies Grants-in-aid from Government of India 1.5 3 Planning and conduct of audit 1.6 3 Significant audit observations and response of Government 1.7 4 to audit Recoveries at the instance of audit 1.8 4 Lack of responsiveness of Government to Audit 1.9 5 Follow-up on Audit Reports 1.10 5 Status of placement of Separate Audit Reports of 1.11 6 autonomous bodies in the State Assembly Year-wise details of reviews and paragraphs appeared in 1.12 7 Audit Report Chapter – 2 Performance Audit Public Health Engineering Department 2.1 9 Sewerage Schemes Urban Local Bodies Department 2.2 27 Working of Urban Local Bodies Education Department (Haryana School Shiksha Pariyojna Parishad) 2.3 46 Sarva Shiksha Abhiyan Rural Development Department 2.4 66 Indira Awaas Yojna Cooperation Department 2.5 80 Working of Cooperation Department Reference to Paragraphs Page Chapter – 3 Compliance Audit Civil Aviation Department Irregularities in the functioning of Civil Aviation 3.1 99 Department Civil Secretariat 3.2 102 Irregular expenditure Allotment of space to banks without execution of agreement 3.3 104 Development and Panchayat Department 3.4 105 Management of panchayat land Food and Supplies Department Loss due to distribution of foodgrains to ineligible ration 3.5 110 card holders Health and Medical -

Residential Plotted Colony “Bestech City” at Sector-7, Dharuhera, Rewari, Haryana
HALF YEARLY COMPLIANCE REPORT OF RESIDENTIAL PLOTTED COLONY “BESTECH CITY” AT SECTOR-7, DHARUHERA, REWARI, HARYANA BY M/s BESTECH INDIA PVT. LTD. ADDRESS: BESTECH HOUSE, PLOT – 124, SECTOR – 44, GURGAON DECEMBER 2015 BOARD OF RESOLUTION CONSULTANT PROFILE & QCI NABET CERTIFICATE COMPLIANCE REPORT Modification & Expansion of Existing Residential Plotted Colony “Bestech City” Village – Malpura, Sector – 7, Dharuhera, Rewari Compliance Report COMPLIANCE REPORT S. No. Conditions Proposal PART A - SPECIFIC CONDITIONS: A. Construction phase 1. “Consent to Establishment” shall be “Consent for Establish” has been obtained obtained from Haryana State Pollution from Haryana State Pollution Control Board Control Board under Air & Water Act & a & the copy of the same is attached as copy shall be submitted to the SEIAA Annexure - I. Haryana before the start of any construction work at site. 2. A first aid room as proposed in the project Adequate first Aid facilities have been report will be provided both during ensured for construction labours. construction and operational phase of the project. 3. Adequate drinking water and sanitary Fresh water will be supplied through private facilities should be provided for water tankers for drinking purpose. construction workers at the site. Provision should be made for mobile toilets. Open Temporary toilets have been provided for the defecation by the laborers is strictly labors. prohibited. The safe disposal of waste water and solid waste generated during the Solid waste generated from the site will be construction phase should be ensured. managed as per the Solid Waste Management (Handling) Rules 2000. 4. All the topsoil excavated during All the topsoil excavated during construction construction activities should be stored for activities is being stored for use in use in horticulture/ landscape development horticulture/ landscape development within within the project site. -

PRESENTATION on DELHI-GURGAON-REWARI-ALWAR RRTS CORRIDOR ALIGNMENT Contents
PRESENTATION ON DELHI-GURGAON-REWARI-ALWAR RRTS CORRIDOR ALIGNMENT Contents Proposed Station Locations Route Comparison Alternative Routes Methodology for Selection of RRTS V/s MRTS Alignment Introduction Introduction • NCRPB) - Horizon 2032 - Identified Eight RRTS (Regional Rapid Transit System) Rail Corridors – Delhi – Gurgaon – Rewari – Alwar (158 Kms) [DGRA - Project Corridor] – Delhi – Ghaziabad – Meerut (67 Kms) – Delhi – Sonipat – Panipat (89 Kms) – Delhi – Faridabad – Ballabgarh – Palwal (60kms) – Delhi – Bahadurgarh – Rohtak (70 Kms) – Delhi – Shahadra – Baraut (56 Kms) – Ghaziabad – Khurja (83 Kms) – Ghaziabad – Hapur (57 Kms) • RRTS corridors development broadly along the existing rail alignments – Deviate where necessary to connect - present and likely growth centers – RRTS to provide a rapid transportation system for intra regional movement RRTS v/s MRTS METRO/ Parameter RRTS MRTS Inter city/ Intra City / Traffic Served Intra Regional Sub urban Station Spacing (Km) 5 + 0.8 to 2 Average Station Spacing (Km) 10 1 Max Speed (KmPH) 160 + 80 Booked Speed (KmPH) 145 + 70 Average Speed (KmPH) 80 - 100 28 - 35 Methodology for Alignment Selection Reconnaissance Survey and Secondary Data Collection Background Case Study of Demographic Studies Suburban Traffic Surveys Projections Systems Travel Demand Forecast Parameters such as •EIA Screening •Landuse Various Alternative Routes •Existing Rly Facilities Ridership Projection •TOD Potential for Alternative Routes Alignment Options DETAILED ALIGNMENT, STATIONS AND TRANSIT ORIENTED DEVELOPMENT -

State District Branch Address Centre Ifsc Contact1 Contact2 Contact3 Micr Code
STATE DISTRICT BRANCH ADDRESS CENTRE IFSC CONTACT1 CONTACT2 CONTACT3 MICR_CODE ANDAMAN NO 26. MG ROAD AND ABERDEEN BAZAR , NICOBAR PORT BLAIR -744101 704412829 704412829 ISLAND ANDAMAN PORT BLAIR ,A & N ISLANDS PORT BLAIR IBKL0001498 8 7044128298 8 744259002 UPPER GROUND FLOOR, #6-5-83/1, ANIL ANIL NEW BUS STAND KUMAR KUMAR ANDHRA ROAD, BHUKTAPUR, 897889900 ANIL KUMAR 897889900 PRADESH ADILABAD ADILABAD ADILABAD 504001 ADILABAD IBKL0001090 1 8978899001 1 1ST FLOOR, 14- 309,SREERAM ENCLAVE,RAILWAY FEDDER ROADANANTAPURA ANDHRA NANTAPURANDHRA ANANTAPU 08554- PRADESH ANANTAPUR ANANTAPUR PRADESH R IBKL0000208 270244 D.NO.16-376,MARKET STREET,OPPOSITE CHURCH,DHARMAVA RAM- 091 ANDHRA 515671,ANANTAPUR DHARMAVA 949497979 PRADESH ANANTAPUR DHARMAVARAM DISTRICT RAM IBKL0001795 7 515259202 SRINIVASA SRINIVASA IDBI BANK LTD, 10- RAO RAO 43, BESIDE SURESH MYLAPALL SRINIVASA MYLAPALL MEDICALS, RAILWAY I - RAO I - ANDHRA STATION ROAD, +91967670 MYLAPALLI - +91967670 PRADESH ANANTAPUR GUNTAKAL GUNTAKAL - 515801 GUNTAKAL IBKL0001091 6655 +919676706655 6655 18-1-138, M.F.ROAD, AJACENT TO ING VYSYA BANK, HINDUPUR , ANANTAPUR DIST - 994973715 ANDHRA PIN:515 201 9/98497191 PRADESH ANANTAPUR HINDUPUR ANDHRA PRADESH HINDUPUR IBKL0001162 17 515259102 AGRICULTURE MARKET COMMITTEE, ANANTAPUR ROAD, TADIPATRI, 085582264 ANANTAPUR DIST 40 ANDHRA PIN : 515411 /903226789 PRADESH ANANTAPUR TADIPATRI ANDHRA PRADESH TADPATRI IBKL0001163 2 515259402 BUKARAYASUNDARA M MANDAL,NEAR HP GAS FILLING 91 ANDHRA STATION,ANANTHAP ANANTAPU 929710487 PRADESH ANANTAPUR VADIYAMPETA UR -

Government of Haryana Department of Revenue & Disaster Management
Government of Haryana Department of Revenue & Disaster Management DISTRICT DISASTER MANAGEMENT PLAN PANCHKULA 2013 Prepared By HARYANA INSTITUTE OF PUBLIC ADMINISTRATION, Plot 76, HIPA Complex, Sector 18, Gurgaon District Disaster Management Plan, Panchkula Panchkula 3 | P a g e District Disaster Management Plan, Panchkula 4 | P a g e District Disaster Management Plan, Panchkula Abbreviations AC Area Commander ACA Additional Central Assistance ADC Additional Deputy Commissioner ADO Agriculture Development Officer AFSO Assistant Food and Supplies Officer AFSO Assistant Fire Station Officer ARWSP Accelerated Rural Water Supply Programme ASHA Accredited Social Health Activist ASI Assistant Sub-Inspectors ATF Aviation Turbine Fuel BAO Block Agriculture Officer BCP Business Continuity Planning BDO Block Development Officer BIS Bureau of Indian Standards BPCL Bharat Petroleum Corporation Limited BPL Below Poverty Line BSNL Bharat Sanchar Nigam Ltd CBDM Community Based Disaster Management CBDRR Community-Based Disaster Risk Reduction CBO Community Based Organisation CBOs Community Based Organisations CBRN Chemical, Biological, Radiological and Nuclear CCMNC Cabinet Committee on Management of Natural Calamities CCS Cabinet Committee on Security CD Civil Defence CDHG Civil Defence & Home Guards CDI Civil Defence Instructor CDM Center for Disaster Management CDRN Corporate Disaster Resource Network CEO Chief Executive Officer CHC Community Health Center CM Chief Minister CMG Crisis Management Group CMO Chief Medical Officer CO Circle Officer Com./CUL -

COMPENDIUM of JUDGMENTS Association for Advocacy and Legal
COMPENDIUM OF JUDGMENTS RIGHT TO CHOICE IN RELATIONSHIPS Association for Advocacy and Legal Initiatives (AALI), 2011 Acknowledgements The compendium of judgments comes out of our work on the issue of Right to Choices since 2000. Bringing the judgments together in a publication was inspired by the National Colloquium organized by AALI in partnership with the UP Institute of Judicial Training and Research. We are very grateful to all the participants and resource team for all their guidance, in-puts and support, Words are not enough to thank our Advisors and Board, for all their support and work, especially: Niti Saxena, for her idea in putting it together, framing this and making it possible, Seema Misra, for the editing, analysis, and all the work, We are also very grateful to Shubhangi Singh our intern and Shubham Tripathi for all their work in putting the Compendium together, However, this would not be ready for publication without all the work done by Somesh Srivastava in ensuring all the re-reading, copy-editing, numbering and all things needed to ensure a publishable version. Finally, we hope that this Compendium is useful for judges and practitioners; as well as those who continue to give meaning to human rights through their struggles, challenges and achievements, We look forward to feedback and comments AALI Team 1 Table of Contents INTRODUCTION ....................................................................................................................................... 4 INDICES .................................................................................................................................................... -
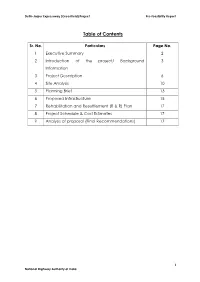
Table of Contents
Delhi-Jaipur Expressway (Greenfield) Project Pre-feasibility Report Table of Contents Sr. No. Particulars Page No. 1 Executive Summary 2 2 Introduction of the project/ Background 3 Information 3 Project Description 6 4 Site Analysis 10 5 Planning Brief 13 6 Proposed Infrastructure 15 7 Rehabilitation and Resettlement (R & R) Plan 17 8 Project Schedule & Cost Estimates 17 9 Analysis of proposal (Final Recommendations) 17 1 National Highway Authority of India Delhi-Jaipur Expressway (Greenfield) Project Pre-feasibility Report PRE-FEASIBILITY REPORT 1. Executive Summary The proposed Greenfield expressway will start at km 40.10 of NH-8 near Kherki Dhaula Toll Plaza and, it will terminate at km 217.0 of NH- 8 near Chandwaji. Total length of project road is 195.10 km. The TOR for initial design was considered in 128th meeting of EAC on 20th-23rd November, 2013 and Terms of references for EIA study was granted by MoEF, New Delhi on 10th December, 2013 vide F. No. 10-62/2013-IA.III In the light of high cost and subsequent decision of Ministry of Road, Transport and Highway and Government of Haryana, the proposed Greenfield Expressway alignment was revised and now it will start at km 40.10 of NH-8 near Kherki Dhaula Toll Plaza and, it will terminate at km 217.0 of NH- 8 near Chandwaji. Total revised length of project road is 195.10 km. The spurs have been deleted in the present proposal and factually the main expressway alignment remains the same except change in start and end point. The project was also presented in the 161st meeting of EAC held on 26th July, 2016 and Minutes of meeting has been displayed on the website of MoEF&CC on 08th August, 2016, where the project proponent was advised “to resubmit the proposal clearly mentioning about the earlier ToRs and a categorical declaration regarding withdrawing the previous proposal or continuing with it, and a clear view on the present one”. -

Communications
CHAPTER VII COMMUNICATIONS INTRODUCTION The communication networks of an area are akin to the circulatory and nervous systems of a body which facilitate trade, transport, social integration, and economic development that particular area. These facilitate extensions and specializations of markets. Just like a body cannot develop properly and fully in the absence of its circulatory and nervous system, an area cannot develop properly and fully in the absence of means of communications. The growth of communications of an area serves an important indicator of the overall development of an area and its society, and hence means of communications in an area are of great importance. A brief account of development of major means of communications in the Jhajjar district is given below under different headings. ROADS AND ROAD TRANSPORT ROADS Prior to 1870, almost fair weather unmetalled roads served for the transport action of grain to the markets. The chief lines of communication through roads were Rohtak to Jhajjar; from Beri towards Bhiwani and to Sampla; and feeder roads from Jhajjar towards Dadri, Kanaund, Pataudi, to Farruknagar and Bahadurgarh. The road of the Custom preventive line, which was removed in 1879, ran athwart the district, from Meham to Badli, through Kalanaur, Kanhaur, Beri and Jhajjar, was kept up, although the line was abolished.1 All the roads were usually in very fair condition, and easy for the traffic of country carts, except after heavy rain. Many of them were strikingly broad, but the heavy traffic of the country carts soon spoiled them and they were often bad for driving and riding alike. -

SR No HOSPITAL NAME
HOSPITAL NAME (Hospitals marked with** S R are Preffered Provider STD Telephon Address City Pin Rohini Code State Zone No Network, with whom ITGI code e has Negotiated package rates) 1 Aasha Hospital** 7-201, Court Road Anantapur 515001 08554 245755 8900080169586 Andhra Pradesh South Zone 2 Aayushman The Family Hospital**45/142A1, V.R. ColonyKurnool 518003 08518 254004 8900080172005 Andhra Pradesh South Zone 3 Akira Eye Hospital** Aryapuram Rajahmundry 533104 0883 2471147 8900080180079 Andhra Pradesh South Zone 4 Andhra Hospitals** C.V.R. Complex, PrakasamVijayawada Road 520002 0866 2574757 8900080172531 Andhra Pradesh South Zone 5 Apex Hospital # 75-6-23, PrakashnagarRajahmundry Rajahmundry 533103 0883 2439191 8900080334724 Andhra Pradesh South Zone 6 Apollo Bgs Hospitals** Adichunchanagari RoadMysore Kuvempunagar 570023 0821 2566666 8900080330627 Karnataka South Zone 7 Apollo Hospital 13-1-3, Suryaraopeta, MainKakinada Road 533001 0884 2379141 8900080341647 Andhra Pradesh South Zone 8 Apollo Hospitals,Vizag Waltair, Main Road Visakhapatnam 530002 0891 2727272 8900080177710 Andhra Pradesh South Zone 9 Apoorva Hospital 50-17-62 Rajendranagar,Visakhapatnam Near Seethammapeta530016 Jn. 0891 2701258 8900080178007 Andhra Pradesh South Zone 10 Aravindam Orthopaedic Physiotherapy6-18-3, KokkondavariCentre Street,Rajahmundry T. Nagar, Rajahmundry533101 East0883 Godavari2425646 8900080179547 Andhra Pradesh South Zone 11 Asram Hospital (Alluri Sitarama N.H.-5,Raju Academy Malaka OfPuram MedicalEluru Sciences)** 534005 08812 249361-62 8900080180895 -

2005-06 to 2009-10
District Development Plan Rewari (2005-06 To 2009-10) Prepared by Additional Deputy Commissioner Rewari CONTENTS Sr. No. Chapter Page No. 1. Introduction 1-13 1.1 History 1-2 1.2 Location and Extent 2 1.3 Administration 2 1.4 Topography 3 1.5 Geology 3 1.6 Climate 3-4 1.7 Temperature 4 1.8 Rainfall 4-5 1.9 Demographic Profile 5-6 1.10 Resource Profile 6-7 1.10.1 Soils 6 1.10.2 Agriculture 6 1.10.3 Hydrology/Hydro morphology 6 1.10.4 Ground Water 6-7 1.11 Drainage and Canals 7-8 1.11.1 Irrigation. 8 1.12 Flora and fauna (Ecology) 8 1.13 Minerals 9 1.14 Natural Hazardous 9 1.15 Live Stock Resource 9 1.16 Industries 9 1.17 Cooperation 9-10 1.17.1 Dairy Cooperatives 10 1.18 Health 10 1.19 Public Health 11 1.20 Infrastructure 11-12 1.21 Revenue 12 1.22 Decentralization /Panchayati Raj Institutions 12 1.23 Description of Blocks 12-13 2. Departmental Activities 14-48 2.1 Agriculture 14-21 2.2 Animal Husbandry and Dairying Department 21-27 2.3.1 Health 27-31 2.3.2 Ayurveda 31-32 2.4.1 Public Works Department (Public Health) 32-38 2.5 Public Works Department (B&R) 38-39 2.6 Education 39-40 2.7 Transport 40-42 2.8.1 Cooperation 42-44 2.8.2 Dairy cooperatives 44 2.9 Rural Development and Poverty Alleviation 44 2.10 Women and Child Development 44-45 2.11 Welfare of Scheduled Castes and Backward Classes 45-47 2.12 Social Justice and Empowerment Department 47-48 3.