Biomaterials in Craniofacial Reconstruction
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Craniofacial Development After Three Different Palatoplasties in Children Born with Isolated Cleft Palate
From the DEPARTMENT OF DENTAL MEDICINE Karolinska Institutet, Stockholm, Sweden CRANIOFACIAL DEVELOPMENT AFTER THREE DIFFERENT PALATOPLASTIES IN CHILDREN BORN WITH ISOLATED CLEFT PALATE Konstantinos A. Parikakis Stockholm 2018 All previously published papers were reproduced with permission from the publisher Published by Karolinska Institutet Printed by Eprint AB 2018 © Konstantinos A. Parikakis, 2018 ISBN 978-91-7831-277-1 Craniofacial development after three different palatoplasties in children born with isolated cleft palate THESIS FOR DOCTORAL DEGREE (Ph.D.) By Konstantinos A. Parikakis Principal Supervisor: Opponent: Associate Professor Agneta Karsten Professor David Rice Karolinska Institutet University of Helsinki Department of Dental Medicine Department of Orthodontics Division of Orthodontics and Pedodontics Examination Board: Co-supervisor(s): Associate Professor Magnus Becker Associate Professor Ola Larson University of Lund Karolinska University Hospital Department of Plastic and Reconstructive Surgery Department of Reconstructive Plastic Surgery Professor Britt Gustafsson Karolinska Institutet Department of Clinical Science, Intervention and Technology (CLINTEC) Division of Pediatrics Associate Professor Farhan Bazargani University of Örebro Centrum för Specialisttandvard Department of Orthodontics To Christina, little Anastasios and…forthcoming Vassilios “Wherever the art of Medicine is loved, there is also a love of Humanity” Hippocrates of Kos, c.460-370 B.C. ABSTRACT Introduction: Different palatoplasties are applied for -

Craniofacial Center
Craniofacial Center The team concept The Craniofacial Center at Children’s Hospital New Orleans is dedicated to providing holistic, coordinated, state-of-the-art care to children with craniofacial differences. All team members specialize in complexities of caring for children with clefts and other craniofacial conditions. Children with clefts and craniofacial differences thrive best when cared for by specialists from many different disciplines. The team approach ensures that healthcare providers work together to implement a single, coordinated, and patient-centered treatment plan unique to your child. Craniofacial Center Craniofacial Pediatrics Genetics Otolaryngology The craniofacial pediatrician will Many babies with craniofacial Our otolaryngologists are surgeons diagnose your child and manage conditions have “isolated” problems with expertise in treating disorders medical problems related to that do not affect their general of the head, neck, ears, nose and their craniofacial differences. The health. The geneticist identifies throat in children of all ages. They physician guides your child’s overall those few patients who may have a assess and monitor your child’s treatment and works with other more complicated genetic condition hearing, ears, feeding, breathing team members to coordinate associated with other medical and speech development. specialty care. Your craniofacial problems and/or family history. They pediatrician will be familiar with all can advise you about the pros and Neurosurgery aspects of your child’s condition and cons of genetic testing, counsel the Neurosurgeons specialize in treating with your family’s needs and desires. family, and give information about children with abnormalities of the The craniofacial pediatrician will the prognosis and recurrence risks. -

A Guide to Safety Protocols for International Craniofacial Outreach
CE: R.R.; SCS-20-0960; Total nos of Pages: 4; SCS-20-0960 SPECIAL EDITORIAL A Guide to Developing Safety Protocols for International Craniofacial Outreach Programs During the COVID-19 Era Parsa P. Salehi, MD,Ã Adam B. Johnson, MD, PhD,y Brian Rubinstein, MD,z Nima Pahlavan, MD, DDS,§ Babak Azizzadeh, MD, FACS,jj and Usama S. Hamdan, MDô procedures to the ‘‘new normal.’’ One important area of health 07/23/2020 on BhDMf5ePHKav1zEoum1tQfN4a+kJLhEZgbsIHo4XMi0hCywCX1AWnYQp/IlQrHD3yRlXg5VZA8ta0m8jqCQrWIIm7WEcSSNRoQmV8QkFTwQ= by https://journals.lww.com/jcraniofacialsurgery from Downloaded Downloaded Abstract: The ongoing COVID-19 outbreak has created obstacles to care delivery that merits attention is the future of craniofacial health care delivery on a global scale. Low- and middle-income outreach programs (CFOP) in the COVID-19 era. from countries (LMICs), many of which already suffered from unmet CFOP provide an essential service to low- and middle-income 1–3 https://journals.lww.com/jcraniofacialsurgery surgical and medical needs, are at great risk of suffering poor health countries (LMICs). Even before the COVID pandemic, the outcomes due to health care access troubles brought on by the surgical needs of LMICs were unmet by existing nongovernmental organizations (NGOs).2 Hence, the pandemic will likely exacerbate pandemic. Craniofacial outreach programs (CFOP)—a staple for 4 craniofacial surgeons—have historically provided essential care to LMICs’ surgical needs. In particular, CFOP are a staple for craniofacial surgeons (which include facial plastic and reconstruc- LMICs. To date, there has not been literature discussing the process of tive surgeons, plastic surgeons, otolaryngologists-head and neck resuming CFOP mission trips. -

Paramedian Mandibular Cleft in a Patient Who Also Had Goldenhar 2
Brief Clinical Studies The Journal of Craniofacial Surgery & Volume 23, Number 1, January 2012 as the thyroid gland and hyoid bone, to determine whether any 10. Franzese C, Hayes JD, Nichols K. Congenital midline cervical cleft: a associated anomalies exist.3,16 Alternatively, CT or magnetic reso- report of two cases. Ear Nose Throat J 2008;87:166Y168 nance imaging may be performed for a more thorough assessment 11. Hirokawa S, Uotani H, Okami H, et al. A case of congenital midline of the soft tissue relationships; in our case, a CT scan of the neck cervical cleft with congenital heart disease. J Pediatr Surg Y confirmed a superficial subcutaneous cord, without deeper tissue 2003;38:1099 1101 involvement. To determine the source of airway obstruction, pre- 12. Tsukuno M, Kita Y, Kurihara K. A case of midline cervical cleft. Congenit Anom (Kyoto) 2002;42:143Y145 operative flexible laryngoscopy should be performed. 13. Vure S, Pang K, Hallam L, et al. Congenital midline cervical cleft Surgical treatment of CMCC is required to alleviate or prevent with an underlying bronchogenic like cyst. Pediatr Surg Int anterior neck contracture, respiratory distress, micrognathia, and 2009;25:811Y813 4,5,13 infection and for aesthetic reasons. Treatment involves the com- 14. Andryk JE, Kerschner JE, Hung RT, et al. Mid-line cervical cleft with a plete excision of the lesion and any involved tissues, followed by bronchogenic cyst. Int J Pediatr Otorhinolaryngol 1999;47:261Y264 closure, which is most commonly performed with a Z-plasty or mul- 15. Agag R, Sacks J, Silver L. -

Lieshout Van Lieshout, M.J.S
EXPLORING ROBIN SEQUENCE Manouk van Lieshout Van Lieshout, M.J.S. ‘Exploring Robin Sequence’ Cover design: Iliana Boshoven-Gkini - www.agilecolor.com Thesis layout and printing by: Ridderprint BV - www.ridderprint.nl ISBN: 978-94-6299-693-9 Printing of this thesis has been financially supported by the Erasmus University Rotterdam. Copyright © M.J.S. van Lieshout, 2017, Rotterdam, the Netherlands All rights reserved. No parts of this thesis may be reproduced, stored in a retrieval system, or transmitted in any form or by any means without permission of the author or when appropriate, the corresponding journals Exploring Robin Sequence Verkenning van Robin Sequentie Proefschrift ter verkrijging van de graad van doctor aan de Erasmus Universiteit Rotterdam op gezag van de rector magnificus Prof.dr. H.A.P. Pols en volgens besluit van het College voor Promoties. De openbare verdediging zal plaatsvinden op woensdag 20 september 2017 om 09.30 uur door Manouk Ji Sook van Lieshout geboren te Seoul, Korea PROMOTIECOMMISSIE Promotoren: Prof.dr. E.B. Wolvius Prof.dr. I.M.J. Mathijssen Overige leden: Prof.dr. J.de Lange Prof.dr. M. De Hoog Prof.dr. R.J. Baatenburg de Jong Copromotoren: Dr. K.F.M. Joosten Dr. M.J. Koudstaal TABLE OF CONTENTS INTRODUCTION Chapter I: General introduction 9 Chapter II: Robin Sequence, A European survey on current 37 practice patterns Chapter III: Non-surgical and surgical interventions for airway 55 obstruction in children with Robin Sequence AIRWAY OBSTRUCTION Chapter IV: Unravelling Robin Sequence: Considerations 79 of diagnosis and treatment Chapter V: Management and outcomes of obstructive sleep 95 apnea in children with Robin Sequence, a cross-sectional study Chapter VI: Respiratory distress following palatal closure 111 in children with Robin Sequence QUALITY OF LIFE Chapter VII: Quality of life in children with Robin Sequence 129 GENERAL DISCUSSION AND SUMMARY Chapter VIII: General discussion 149 Chapter IX: Summary / Nederlandse samenvatting 169 APPENDICES About the author 181 List of publications 183 Ph.D. -

Pediatric/Craniofacial
PEDIATRIC/CRANIOFACIAL Counterclockwise Craniofacial Distraction Osteogenesis for Tracheostomy-Dependent Children with Treacher Collins Syndrome Richard A. Hopper, M.D., Background: The craniofacial rotation deformity in Treacher Collins syndrome M.Sc. results in airway compression that is not addressed by isolated mandibular Hitesh Kapadia, D.D.S., distraction osteogenesis. Our purpose is to present a surgical technique— Ph.D. counterclockwise craniofacial distraction osteogenesis—that improves airway Srinivas Susarla, D.M.D., morphology and occlusal rotation in tracheostomy-dependent patients with M.D., M.P.H. this condition. Randall Bly, M.D. Methods: All patients underwent subcranial Le Fort II osteotomies with simultane- Kaalan Johnson, M.D. ous mandibular osteotomies, followed by coordinated maxillomandibular distrac- Seattle, Wash. tion with counterclockwise rotation. We reviewed pretreatment, posttreatment, and end-treatment cephalograms. Airway changes were assessed using polysom- nography, sleep endoscopy, and direct laryngoscopy. Bivariate statistics were com- puted to compare pretreatment and posttreatment measures. Results: Five subjects (age range, 4.5 to 12.1 years) underwent this new pro- cedure; three had previously undergone mandibular distraction. The average palatal plane rotation was 17 degrees, the effective mandible length increase was 18 mm, and the facial plane relative to skull base rotation was 14 degrees. There was a symmetric 30 percent relapse of rotation with maintained occlusion in the SUPPLEMENTAL DIGITAL CONTENT IS AVAIL- first 9 months of follow-up that then stabilized. Four patients were successfully ABLE IN THE TEXT. decannulated following counterclockwise craniofacial distraction osteogenesis following polysomnography. Sleep endoscopy available on two patients demon- strated resolution of the upper airway obstruction. Conclusions: Counterclockwise craniofacial distraction osteogenesis provided greater palatal rotation than previous techniques. -

Fat Grafting in Orthognathic Surgery
SPECIAL EDITORIAL Fat Grafting in Orthognathic Surgery Rajiv J. Iyengar, MD, Kyle Gabrick, MD, Karl Bruckman, MD, DDS, and Derek M. Steinbacher, MD, DMD at grafting is a powerful tool in plastic surgery. The evolution of Background: Fat grafting is widely utilized in craniofacial surgery. F this technique is fascinating and has been described exten- The authors describe a series of consecutive patients who under- sively.1 At present, the broad procedural practice standards are went orthognathic surgery with fat grafting by the senior author and derived from the work of Coleman.1–5 The so-called lipostructure review relevant literature in the field; fat grafting technique is method involves harvesting fat using a 3-mm blunt cannula with a discussed in detail. The authors also highlight 3 patients to illustrate 10 mL syringe at low negative pressure to minimize adipocyte trauma; postoperative outcomes. Coleman advocates for low RPM centrifugation which allows for Methods: A retrospective cohort of consecutive orthognathic atraumatic separation of oily,aqueous, and fat components. Placement surgery patients was reviewed. Age, sex, BMI, procedure, area then involves the use of an 18-G cannula in the recipient site. Further of harvest, location of injection, donor site complications, and need technical descriptions are included later in this manuscript. The purpose of this paper is to review the benefits of fat grafting for repeat fat grafting were analyzed. Inclusion criteria included during orthognathic surgery; more specifically, we will delineate history of orthognathic surgery and concomitant fat grafting the biology, harvesting, processing, and technical considerations to performed by the senior author in 2015. -

Craniofacial Surgery Hs-268
l CRANIOFACIAL SURGERY HS-268 M i ssour i Care ‘Ohana Health Plan, a plan offered by WellCare Health Insurance of Arizona Staywell of Florida Craniofacial Surgery Children’s M edical Services Health Plan (CMS Health Plan) Policy Number: HS-268 WellCare (Alabama, Arizona, Arkansas, California, Connecticut, Florida, Georgia, Illinois, Indiana, Louisiana, M aine, Michigan, Mississippi, Missouri, New Hampshire, New Original Effective Date: 11/6/2014 Jersey, New York, North Carolina, Ohio, South Carolina, Tennessee, Texas, Washington) Revised Date(s): 8/6/2015; 9/27/2016; WellCare Prescription Insurance 7/6/2017; 6/7/2018; 3/7/2019, 9/5/2019 APPLICATION STATEMENT The application of the Clinical Coverage Guideline is subject to the benefit determinations set forth by the Centers for Medicare and Medicaid Services (CMS) National and Local Cov erage Determinations and state-specific Medicaid mandates, if any. DISCLAIMER The Clinical Coverage Guideline (CCG) is intended to supplement certain standard WellCare benefit plans and aid in administering benefits. Federal and state law, contract language, etc. take precedence over the CCG (e.g., Centers for Medicare and Medicaid Services [CMS] National Coverage Determinations [NCDs], Local Coverage Determinations [LCDs] or other published documents). The terms of a member’s particular Benefit Plan, Evidence of Coverage, Certificate of Coverage, etc., may differ significantly from this Coverage Position. For example, a member’s benefit plan may contain specific exclusions related to the topic addressed in this CCG. Additionally, CCGs relate exclusively to the administration of health benefit plans and are NOT recommendations for treatment, nor should they be used as treatment guidelines. -

Craniofacial Surgery
Craniofacial Surgery: Frequently Asked Questions What condition does craniofacial surgery address? What is the expected surgical outcome for craniosynostosis? Craniofacial surgery is a pediatric surgical subspecialty that involves the reconstructive treatment of disorders of the face and Using modern surgical techniques, studies have shown that craniosynostosis can be corrected with positive outcomes skull. Craniosynostosis is a rare condition in which a baby develops or is born with an abnormally shaped skull. It is caused by and relatively low morbidity and mortality. This is particularly true for otherwise healthy, non-syndromic infants. (Most premature closing of one or multiple cranial sutures (skull bones) on a baby’s head. Babies are born with oating skull craniosynostosis cases are non-syndromic. In other words, not genetically caused.) However, the right surgical team is bones in order to allow them to come through the birth canal and to allow for rapid head growth during the rst year of key. This team largely relies on the pediatric neurosurgeon and plastic surgeon specifically trained in craniofacial procedures. life. Craniosynostosis can be diagnosed as early as birth, however most infants are diagnosed in the rst few months. ANA has renowned expertise in this area and has combined forces to produce the Craniofacial Specialists of New Jersey. Craniosynostosis leads to a restriction in the growth of the skull, which can cause unusual head shape and facial features. In unique cases, it can also cause damage to the brain due to increased pressure inside the skull. The cause of craniosynostosis is unknown. However, in extremely rare cases, craniosynostosis can be inherited and part of a genetic syndrome. -
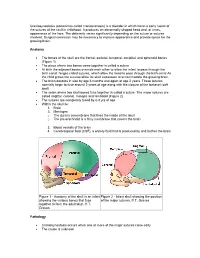
Craniosynostosis (Sometimes Called Craniostenosis) Is a Disorder in Which There Is Early Fusion of the Sutures of the Skull in Childhood
Craniosynostosis (sometimes called craniostenosis) is a disorder in which there is early fusion of the sutures of the skull in childhood. It produces an abnormally shaped head and, at times, appearance of the face. The deformity varies significantly depending on the suture or sutures involved. Surgical correction may be necessary to improve appearance and provide space for the growing brain. Anatomy • The bones of the skull are the frontal, parietal, temporal, occipital, and sphenoid bones (Figure 1) • The place where two bones come together is called a suture • At birth the adjacent bones override each other to allow the infant to pass through the birth canal. hinges called sutures, which allow the head to pass through the birth canal As the child grows the sutures allow for skull expansion to accommodate the growing brain. • The brain doubles in size by age 6 months and again at age 2 years. These sutures normally begin to fuse around 2 years of age along with the closure of the fontanel (soft spot) • The seam where two skull bones fuse together is called a suture. The major sutures are called sagittal, coronal, metopic and lambdoid (Figure 2) • The sutures are completely fused by 6-8 yrs of age • Within the skull lie: 1. Brain 2. Meninges o The dura is a membrane that lines the inside of the skull o The pia-arachnoid is a filmy membrane that covers the brain 3. Blood vessels of the brain 4. Cerebrospinal fluid (CSF), a watery fluid that is produced by and bathes the brain Figure 1 - Anatomy of the skull in an infant Figure 2 - Infant skull showing the position showing the various bones that fuse of the major sutures. -

The Cleft-Craniofacial Center at Albany Med What We Treat
The Cleft-Craniofacial Center at Albany Med What we treat: Cleft-Craniofacial Team at Albany Med The Cleft-Craniofacial Center at Albany Med is a nationally accredited center by the American Cleft Palate-Craniofacial Association (ACPA) and the only center of its kind in Northeastern New York. This comprehensive center is comprised of a team of experts who treat the full spectrum of cleft and craniofacial conditions, from birth to adulthood. The Cleft-Craniofacial Center is a regional Aidan—Cleft lip & palate resource where families can receive care for their children with treating specialists they need in one place during each visit—plastic surgery, dentistry, genetics, neurosurgery, nutrition, orthodontics, otolaryngology (ENT), psychology, and speech language pathology. The mission of the Cleft-Craniofacial Center at Albany Med is to offer world-class care to patients with these congenital differences in a compassionate and convenient setting, Ryder—Craniosynostosis close to home. What we treat: The experts at the Cleft-Craniofacial Center at Albany Med specialize in the services and treatment of the following conditions: Abnormal head Shape/plagiocephaly – Facial asymmetry or facial paralysis flat areas on head Facial trauma and reconstruction Alveolar defect (bone grafting) – bony gap in the gumline/ridge that contain Hemifacial microsomia – asymmetric the sockets of the teeth growth of one side of the face Brachial plexus reconstruction – nerve Jaw deformities – Micrognathia (small), injury resulting from birth or other trauma retrognathia (posterior position), malocclusion (abnormal bite) Burn reconstruction Reconstruction techniques include upper and lower jaw surgery, mandibular Cleft lip – an opening in the upper lip Distraction, etc. Cleft palate – an opening between the Nasal reconstruction (rhinoplasty) – for mouth and nose arrhinia-absent nose, cleft deformity, etc. -

Orthognathic Surgery
ESSENTIALS of Maxillofacial Surgery 2nd edition The American Society of Maxillofacial Surgeons is the oldest American organization representing maxillofacial surgeons who are devoted to improving and promoting the highest level of patient care. Maxillofacial and Craniofacial surgeons specialize in bone and soft tissue repair and reconstruction for enhancement of the face. The Society’s mission is to advance the science and practice of surgery of the facial region and craniofacial skeleton. Continually updated information about the various procedures in maxillofacial surgery, members of the Society, and current issues and news about the specialty can be found at the ASMS website. WWW.MAXFACE.ORG ESSENTIALS OF MAXILLOFACIAL SURGERY 2nd Edition Fan Liang, MD Assistant Professor of Surgery Division of Plastic, Reconstructive and Maxillofacial Surgery R Adams Cowley Shock Trauma Center University of Maryland Sanjay Naran, MD Pediatric Plastic and Craniofacial Surgeon Division of Pediatric Plastic Surgery Advocate Children’s Hospital, Chicago, IL Clinical Instructor, Department of Plastic Surgery University of Pittsburgh School of Medicine Editors Fan Liang, MD Sanjay Naran, MD Contributors Angelo Leto Barone, MD Katelyn Bennett, MD Jessica Ching, MD Russell Ettinger, MD Daniel Gould, MD Thomas Imahiyerobo Jr., MD Graham Ives, MD Breanna Jedrzejewski, MPH, MD Keli Kolegraff, MD Cassie Ligh, MD Meghan McCullough, MD Naikhoba Munabi, MD J. Thomas Paliga, MD Sameer Shakir, MD Todd Thurston, MD, MS Leo Urbinelli, MD, MA Christian Vercler, MD Howard Wang, MD Jason Wink, MD, MSc Jason W Yu, DMD, MD Copyright © 2019 by the American Society of Maxillofacial Surgeons 444 East Algonquin Road Arlington Heights, IL 60005 1st Edition, 2005 Ed.