Pharmacist's Letter Statistics Review
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Statistical Significance of Randomized Controlled Trial Results Is Frequently Fragile: a Case for a Fragility Index
The statistical significance of randomized controlled trial results is frequently fragile: A case for a Fragility Index Walsh, M., Srinathan, S. K., McAuley, D. F., Mrkobrada, M., Levine, O., Ribic, C., Molnar, A. O., Dattani, N. D., Burke, A., Guyatt, G., Thabane, L., Walter, S. D., Pogue, J., & Devereaux, P. J. (2014). The statistical significance of randomized controlled trial results is frequently fragile: A case for a Fragility Index. Journal of Clinical Epidemiology, 67(6), 622-628. https://doi.org/10.1016/j.jclinepi.2013.10.019 Published in: Journal of Clinical Epidemiology Document Version: Publisher's PDF, also known as Version of record Queen's University Belfast - Research Portal: Link to publication record in Queen's University Belfast Research Portal Publisher rights 2014 The Authors. Published by Elsevier Inc. This is an open access article published under a Creative Commons Attribution-NonCommercial-NoDerivs License (https://creativecommons.org/licenses/by-nc-nd/3.0/), which permits distribution and reproduction for non-commercial purposes, provided the author and source are cited. General rights Copyright for the publications made accessible via the Queen's University Belfast Research Portal is retained by the author(s) and / or other copyright owners and it is a condition of accessing these publications that users recognise and abide by the legal requirements associated with these rights. Take down policy The Research Portal is Queen's institutional repository that provides access to Queen's research output. Every effort has been made to ensure that content in the Research Portal does not infringe any person's rights, or applicable UK laws. -
EBM Cheat Sheets for Treatment and Harm Duke Program on Teaching
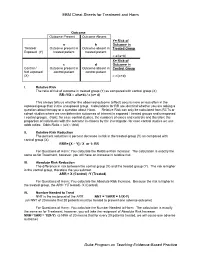
EBM Cheat Sheets for Treatment and Harm Outcome Outcome Present Outcome Absent Y= Risk of a b Outcome in Treated/ Outcome present in Outcome absent in Treated Group Exposed (Y) treated patient treated patient = a/(a+b) X= Risk of c d Outcome in Control / Outcome present in Outcome absent in Control Group Not exposed control patient control patient (X) = c/(c+d) I. Relative Risk The ratio of risk of outcome in treated group (Y) as compared with control group (X) RR=Y/X = a/(a+b) / c (c+ d) This always tells us whether the observed outcome (effect) occurs more or less often in the exposed group than in the unexposed group. Calculations for RR are identical whether you are asking a question about therapy or a question about Harm. Relative Risk can only be calculated from RCTs or cohort studies where we can determine outcomes of interest in exposed / treated groups and unexposed / control groups. (Note: for case control studies, the numbers of cases and controls and therefore the proportion of individuals with the outcome is chosen by the investigator- for case control studies we use odds ratios: Odds Ratio = (a/c) / (b/d) II. Relative Risk Reduction The percent reduction is percent decrease in risk in the treated group (Y) as compared with control group (X) RRR= [X – Y] / X or 1- RR For Questions of Harm: You calculate the Relative Risk Increase: The calculation is exactly the same as for Treatment, however, you will have an increase in relative risk. III. Absolute Risk Reduction The difference in risk between the control group (X) and the treated group (Y). -

Why Do You Need a Biostatistician? Antonia Zapf1* , Geraldine Rauch2 and Meinhard Kieser3
Zapf et al. BMC Medical Research Methodology (2020) 20:23 https://doi.org/10.1186/s12874-020-0916-4 DEBATE Open Access Why do you need a biostatistician? Antonia Zapf1* , Geraldine Rauch2 and Meinhard Kieser3 Abstract The quality of medical research importantly depends, among other aspects, on a valid statistical planning of the study, analysis of the data, and reporting of the results, which is usually guaranteed by a biostatistician. However, there are several related professions next to the biostatistician, for example epidemiologists, medical informaticians and bioinformaticians. For medical experts, it is often not clear what the differences between these professions are and how the specific role of a biostatistician can be described. For physicians involved in medical research, this is problematic because false expectations often lead to frustration on both sides. Therefore, the aim of this article is to outline the tasks and responsibilities of biostatisticians in clinical trials as well as in other fields of application in medical research. Keywords: Medical research, Biostatistician, Tasks, Responsibilities Background Biometric Society (IBS) provides a definition of biomet- What is a biostatistician, what does he or she actually do rics as a ‘field of development of statistical and mathem- and what distinguishes him or her from, for example, an atical methods applicable in the biological sciences’ [2]. epidemiologist? If we would ask this our main cooper- In here, we will focus on (human) medicine as area of ation partners like physicians or biologists, they probably application, but the results can be easily transferred to could not give a satisfying answer. This is problematic the other biological sciences like, for example, agricul- because false expectations often lead to frustration on ture or ecology. -

NUMBER NEEDED to TREAT (CONTINUATION) Confidence
Number Needed to treat Confidence Interval for NNT • As with other estimates, it is important that the uncertainty in the estimated number needed to treat is accompanied by a confidence interval. • A common method is that proposed by Cook and Sacket (1995) and based on calculating first the confidence interval NUMBER NEEDED TO TREAT for the difference between the two proportions. • The result of this calculation is 'inverted' (ie: 1/CI) to give a (CONTINUATION) confidence interval for the NNT. Hamisu Salihu, MD, PhD Confidence Interval for NNT Confidence Interval for NNT • This computation assumes that ARR estimates from a sample of similar trials are normally distributed, so we can use the 95th percentile point from the standard normal distribution, z0.95 = 1.96, to identify the upper and lower boundaries of the 95% CI. Solution Class Exercise • In a parallel clinical trial to test the benefit of additional anti‐ hyperlipidemic agent to standard protocol, patients with acute Myocardial Infarction (MI) were randomly assigned to the standard therapy or the new treatment that contains standard therapy and simvastatin (an HMG‐CoA reductase inhibitor). At the end of the study, of the 2223 patients in the control group 622 died as compared to 431 of 2221 patients in the Simvastatin group. – Calculate the NNT and the 95% CI for Simvastatin – Is Simvastatin beneficial as additional therapy in acute cases of MI? 1 Confidence Interval Number Needed to for NNT Treat • The NNT to prevent one additional death is 12 (95% • A negative NNT indicates that the treatment has a CI, 9‐16). -

A Number-Needed-To-Treat Analysis of the EMAX Trial
Efficacy of Umeclidinium/Vilanterol Versus Umeclidinium or Salmeterol: A Number-Needed-to-Treat Analysis of the EMAX Trial Poster No. 1091 (A3326) 1 2 3 1 2 3 4 5 Respiratory Medicine and Allergology, Lund University, Lund, Sweden; Centre de Pneumologie, Institut Universitaire de Cardiologie et de Pneumologie de Québec, Université Laval, QC, Canada; Department of Medicine, Pulmonary and Critical Care Medicine, University Medical Center Giessen and Marburg, Bjermer L , Maltais F , Vogelmeier CF , Naya I , Jones PW , 4 5 6 6 5 5 7 8 9 Philipps-Universität Marburg, Germany, Member of the German Center for Lung Research (DZL); Global Specialty & Primary Care, GSK, Brentford, Middlesex, UK (at the time of the study) and RAMAX Ltd., Bramhall, Cheshire, UK; GSK, Brentford, Middlesex, UK; Precise Approach Ltd, contingent worker on assignment at Tombs L , Boucot I , Compton C , Lipson DA , Keeley T , Kerwin E GSK, Stockley Park West, Uxbridge, Middlesex, UK; 7Respiratory Clinical Sciences, GSK, Collegeville, PA, USA and Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA; 8GSK, Stockley Park West, Uxbridge, Middlesex, UK; 9Clinical Research Institute of Southern Oregon, Medford, OR, USA Background Results ● The relative treatment benefits with long-acting muscarinic antagonist/long-acting Patients β -agonist (LAMA/LABA) combination therapy versus LAMA or LABA monotherapy have not been Table 1. Patient demographics and clinical characteristics Figure 2. Hazard ratio for a first moderate/severe exacerbation or CID at Week 24 2 ● Demographics and baseline characteristics are shown in Table 1. demonstrated in symptomatic, inhaled corticosteroid (ICS)-free patients with COPD who have a low UMEC/VI vs UMEC UMEC/VI vs SAL UMEC/VI UMEC SAL risk of exacerbations. -

Medical Statistics PDF File 9
M249 Practical modern statistics Medical statistics About this module M249 Practical modern statistics uses the software packages IBM SPSS Statistics (SPSS Inc.) and WinBUGS, and other software. This software is provided as part of the module, and its use is covered in the Introduction to statistical modelling and in the four computer books associated with Books 1 to 4. This publication forms part of an Open University module. Details of this and other Open University modules can be obtained from the Student Registration and Enquiry Service, The Open University, PO Box 197, Milton Keynes MK7 6BJ, United Kingdom (tel. +44 (0)845 300 60 90; email [email protected]). Alternatively, you may visit the Open University website at www.open.ac.uk where you can learn more about the wide range of modules and packs offered at all levels by The Open University. To purchase a selection of Open University materials visit www.ouw.co.uk, or contact Open University Worldwide, Walton Hall, Milton Keynes MK7 6AA, United Kingdom for a brochure (tel. +44 (0)1908 858779; fax +44 (0)1908 858787; email [email protected]). Note to reader Mathematical/statistical content at the Open University is usually provided to students in printed books, with PDFs of the same online. This format ensures that mathematical notation is presented accurately and clearly. The PDF of this extract thus shows the content exactly as it would be seen by an Open University student. Please note that the PDF may contain references to other parts of the module and/or to software or audio-visual components of the module. -

The Use of Stata in Medical Statistics and Epidemiology: a Long Journey
A retrospective view Stata Progress Epidemiology and Biostatistics with Stata My Journey with Stata The use of Stata in Medical Statistics and Epidemiology: a long journey Rino Bellocco, Sc.D. Department of Statistics and Quantitative Methods University of Milano-Bicocca & Department of Medical Epidemiology and Biostatistics Karolinska Institutet Nordic and Baltic Stata Users Group meeting, Stockholm, September 1, 2017 1 / 44 A retrospective view Stata Progress Epidemiology and Biostatistics with Stata My Journey with Stata Outline A retrospective view Stata Progress Epidemiology and Biostatistics with Stata My Journey with Stata 2 / 44 A retrospective view Stata Progress Epidemiology and Biostatistics with Stata My Journey with Stata Outline A retrospective view Stata Progress Epidemiology and Biostatistics with Stata My Journey with Stata 3 / 44 A retrospective view Stata Progress Epidemiology and Biostatistics with Stata My Journey with Stata Some History 1985-1995 • Version 1.0 - 1.5, January 1985 - February 1987 (around 48 commands, regress logit ) 4 / 44 N. J. Cox 15 Table 2: Releases of Stata 1.0 January 1985 3.1 August 1993 1.1 February 1985 4.0 January 1995 1.2 March 1985 5.0 October 1996 1.4 August 1986 6.0 January 1999 1.5 February 1987 7.0 December 2000 2.0 June 1988 8.0 January 2003 A retrospective view Stata2.05 Progress June 1989Epidemiology and Biostatistics8.1 July with 2003 Stata My Journey with Stata 2.1 September 1990 8.2 October 2003 3.0 MarchBasic 1992 Commands Table 3: Stata 1.0 and Stata 1.1 append dir infile plot spool beep do input query summarize by drop label regress tabulate capture erase list rename test confirm exit macro replace type convert expand merge run use correlate format modify save count generate more set describe help outfile sort Stata 1.0 had int, long, float,anddouble variables; it did not have byte or str#. -

Routine Circumcision of Newborn Infants Re
A Trade-off Analysis of Routine Newborn Circumcision Dimitri A. Christakis, MD, MPH*‡; Eric Harvey, PharmD, MBA‡; Danielle M. Zerr, MD, MPH*; Chris Feudtner, MD, PhD*§; Jeffrey A. Wright, MD*; and Frederick A. Connell, MD, MPH‡i Abstract. Background. The risks associated with outine circumcision of newborn infants re- newborn circumcision have not been as extensively mains controversial.1–3 Although a recent evaluated as the benefits. Rpolicy statement by the American Academy Objectives. The goals of this study were threefold: 1) of Pediatrics recommended that parents be given to derive a population-based complication rate for new- accurate and unbiased information regarding the born circumcision; 2) to calculate the number needed to risks and benefits of the procedure,3 accomplishing harm for newborn circumcision based on this rate; and this task of well informed consent is hindered both 3) to establish trade-offs based on our complication rates and published estimates of the benefits of circumcision by the lack of precise information about potential including the prevention of urinary tract infections and harm and by the lack of clear ways to present this penile cancer. information. Methods. Using the Comprehensive Hospital Ab- The benefits of circumcision have been described stract Reporting System for Washington State, we retro- in numerous previous studies using a variety of spectively examined routine newborn circumcisions methodologies.4–8 Reported benefits include reduc- performed over 9 years (1987–1996). We used Interna- tion in the risk of penile cancer,9 urinary tract in- tional Classification of Diseases, Ninth Revision codes fections (UTIs),4,5 and sexually transmitted diseas- to identify both circumcisions and complications and es.10,11 Although the extent to which circumcision limited our analyses to children without other surgical decreases the risk of each of these outcomes has procedures performed during their initial birth hospi- been debated,6,12–15 a consensus appears to be talization. -

Medical Statistics Made Easy Medical Statistics Made Easy M
MEDICAL STATISTICS MADE EASY STATISTICS MEDICAL MEDICAL STATISTICS MADE EASY M. HARRIS and G. TAYLOR 4th EDITION HARRIS and TAYLOR 4 MEDICAL STATISTICS MADE EASY 4 MSME_Book.indb 1 17/08/2020 12:10 MSME_4e_234x156_Prelims.indd 1 06/05/2020 16:02 MEDICAL STATISTICS MADE EASY 4 Michael Harris Professor of Primary Care and former General Practitioner, Bath, UK and Gordon Taylor Professor of Medical Statistics, College of Medicine and Health, University of Exeter, UK MSME_Book.indb 3 17/08/2020 12:10 MSME_4e_234x156_Prelims.indd 3 06/05/2020 16:02 Fourth edition © Scion Publishing Ltd, 2021 ISBN 978 1 911510 63 5 Third edition published in 2014 by Scion Publishing Ltd (978 1 907904 03 5) Second edition published in 2008 by Scion Publishing Ltd (978 1 904842 55 2) First edition published in 2003 by Martin Dunitz (1 85996 219 X) Scion Publishing Limited The Old Hayloft, Vantage Business Park, Bloxham Road, Banbury OX16 9UX, UK www.scionpublishing.com Important Note from the Publisher The information contained within this book was obtained by Scion Publishing Ltd from sources believed by us to be reliable. However, while every effort has been made to ensure its accuracy, no responsibility for loss or injury whatsoever occasioned to any person acting or refraining from action as a result of information contained herein can be accepted by the authors or publishers. Readers are reminded that medicine is a constantly evolving science and while the authors and publishers have ensured that all dosages, applications and practices are based on current indications, there may be specific practices which differ between communities. -

History of Health Statistics
certain gambling problems [e.g. Pacioli (1494), Car- Health Statistics, History dano (1539), and Forestani (1603)] which were first of solved definitively by Pierre de Fermat (1601–1665) and Blaise Pascal (1623–1662). These efforts gave rise to the mathematical basis of probability theory, The field of statistics in the twentieth century statistical distribution functions (see Sampling Dis- (see Statistics, Overview) encompasses four major tributions), and statistical inference. areas; (i) the theory of probability and mathematical The analysis of uncertainty and errors of mea- statistics; (ii) the analysis of uncertainty and errors surement had its foundations in the field of astron- of measurement (see Measurement Error in omy which, from antiquity until the eighteenth cen- Epidemiologic Studies); (iii) design of experiments tury, was the dominant area for use of numerical (see Experimental Design) and sample surveys; information based on the most precise measure- and (iv) the collection, summarization, display, ments that the technologies of the times permit- and interpretation of observational data (see ted. The fallibility of their observations was evident Observational Study). These four areas are clearly to early astronomers, who took the “best” obser- interrelated and have evolved interactively over the vation when several were taken, the “best” being centuries. The first two areas are well covered assessed by such criteria as the quality of obser- in many histories of mathematical statistics while vational conditions, the fame of the observer, etc. the third area, being essentially a twentieth- But, gradually an appreciation for averaging observa- century development, has not yet been adequately tions developed and various techniques for fitting the summarized. -

Are Randomized Double-Blind Experiments Everything?
Int. Statistical Inst.: Proc. 58th World Statistical Congress, 2011, Dublin (Session CPS056) p.5278 Are Randomized Double-blind Experiments Everything? Hampel, Frank Swiss Federal Institute of Technology (ETH), Dept. of Mathematics CH-8092 Zurich, Switzerland E-mail: [email protected] 1. Introduction and overview In medical statistics, randomized double-blind experiments are one of the most important tools. In order to test a new drug or treatment, it is applied to a randomly selected subgroup of patients, while the others obtain a comparison treatment or no treatment at all. Single blinding means that the patient does not know what he or she gets, and double blinding means that also the doctor who judges the effect does not know what has been applied. Obviously, this is to avoid any subjective biases, and it is necessary if the results are not clearcut. And the randomizing ensures that \in the long run" any significant differences are due to the treatment effects. Because of these nice properties, some medical statisticians strongly demand that all evidence should only come from such experiments, they consider it a panacea and seem to tend to ignore all other information. However, apart from this limited view, there are also some problems with this approach. One is obviously that the law of large numbers may not yet be applicable. To avoid this, bigger and bigger experiments are proposed, and/or many smaller experiments are combined in a meta-analysis. One danger is that the quality and homogeneity is much harder to ensure in big experiments, even more so in (tendentiously heterogeneous) parts of a meta-analysis. -

Medical Statistics
community project encouraging academics to share statistics support resources All stcp resources are released under a Creative Commons licence stcp-rothwell-types_of_trials The following resources are associated: Statistical Hypothesis testing, Medical Statistics Types of Trials There are a number of different ways that a hypothesis can be investigated and this sheet aims to provide an overview of the various trial types that can be conducted. The terms factor or hazard are used in this sheet and these are classed as exposures. For example, consider exposure to second-hand cigarette smoke, or intense sun exposure as a hazard or factor. They are something that could cause harm and are generally the factor which the researcher is interested in investigating. Randomised Controlled Trials Randomised controlled trials (RCTs) are the typical clinical trial or research study used to test a particular intervention (such as a drug or diet). Generally there is an experimental intervention of interest being tested against a standard intervention, often referred to as a control (such as current treatment or usual care), or a placebo (an intervention with no active properties). The format of these RCTs can vary, but there are two main types which are parallel group trials and cross over trials. Parallel Group Trials In a parallel group trial patients are randomly assigned to one of two treatment groups. The ideal scenario is that all other baseline characteristics of the patients are roughly similar in each group (i.e. equal number of males and females, location of hospital, patient ages, etc.). Randomisation of patients to treatment allocation is used to try to balance out the patient characteristics and this can be stratified by the characteristics that are deemed to be the most important or influential.