50 Years of Pharmacovigilance: Unfinished Job Joan-Ramon Laporte
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Descriptive Statistics (Part 2): Interpreting Study Results
Statistical Notes II Descriptive statistics (Part 2): Interpreting study results A Cook and A Sheikh he previous paper in this series looked at ‘baseline’. Investigations of treatment effects can be descriptive statistics, showing how to use and made in similar fashion by comparisons of disease T interpret fundamental measures such as the probability in treated and untreated patients. mean and standard deviation. Here we continue with descriptive statistics, looking at measures more The relative risk (RR), also sometimes known as specific to medical research. We start by defining the risk ratio, compares the risk of exposed and risk and odds, the two basic measures of disease unexposed subjects, while the odds ratio (OR) probability. Then we show how the effect of a disease compares odds. A relative risk or odds ratio greater risk factor, or a treatment, can be measured using the than one indicates an exposure to be harmful, while relative risk or the odds ratio. Finally we discuss the a value less than one indicates a protective effect. ‘number needed to treat’, a measure derived from the RR = 1.2 means exposed people are 20% more likely relative risk, which has gained popularity because of to be diseased, RR = 1.4 means 40% more likely. its clinical usefulness. Examples from the literature OR = 1.2 means that the odds of disease is 20% higher are used to illustrate important concepts. in exposed people. RISK AND ODDS Among workers at factory two (‘exposed’ workers) The probability of an individual becoming diseased the risk is 13 / 116 = 0.11, compared to an ‘unexposed’ is the risk. -

Limitations of the Odds Ratio in Gauging the Performance of a Diagnostic, Prognostic, Or Screening Marker
American Journal of Epidemiology Vol. 159, No. 9 Copyright © 2004 by the Johns Hopkins Bloomberg School of Public Health Printed in U.S.A. All rights reserved DOI: 10.1093/aje/kwh101 Limitations of the Odds Ratio in Gauging the Performance of a Diagnostic, Prognostic, or Screening Marker Margaret Sullivan Pepe1,2, Holly Janes2, Gary Longton1, Wendy Leisenring1,2,3, and Polly Newcomb1 1 Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA. 2 Department of Biostatistics, University of Washington, Seattle, WA. 3 Division of Clinical Research, Fred Hutchinson Cancer Research Center, Seattle, WA. Downloaded from Received for publication June 24, 2003; accepted for publication October 28, 2003. A marker strongly associated with outcome (or disease) is often assumed to be effective for classifying persons http://aje.oxfordjournals.org/ according to their current or future outcome. However, for this assumption to be true, the associated odds ratio must be of a magnitude rarely seen in epidemiologic studies. In this paper, an illustration of the relation between odds ratios and receiver operating characteristic curves shows, for example, that a marker with an odds ratio of as high as 3 is in fact a very poor classification tool. If a marker identifies 10% of controls as positive (false positives) and has an odds ratio of 3, then it will correctly identify only 25% of cases as positive (true positives). The authors illustrate that a single measure of association such as an odds ratio does not meaningfully describe a marker’s ability to classify subjects. Appropriate statistical methods for assessing and reporting the classification power of a marker are described. -

Guidance for Industry E2E Pharmacovigilance Planning
Guidance for Industry E2E Pharmacovigilance Planning U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation and Research (CBER) April 2005 ICH Guidance for Industry E2E Pharmacovigilance Planning Additional copies are available from: Office of Training and Communication Division of Drug Information, HFD-240 Center for Drug Evaluation and Research Food and Drug Administration 5600 Fishers Lane Rockville, MD 20857 (Tel) 301-827-4573 http://www.fda.gov/cder/guidance/index.htm Office of Communication, Training and Manufacturers Assistance, HFM-40 Center for Biologics Evaluation and Research Food and Drug Administration 1401 Rockville Pike, Rockville, MD 20852-1448 http://www.fda.gov/cber/guidelines.htm. (Tel) Voice Information System at 800-835-4709 or 301-827-1800 U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation and Research (CBER) April 2005 ICH Contains Nonbinding Recommendations TABLE OF CONTENTS I. INTRODUCTION (1, 1.1) ................................................................................................... 1 A. Background (1.2) ..................................................................................................................2 B. Scope of the Guidance (1.3)...................................................................................................2 II. SAFETY SPECIFICATION (2) ..................................................................................... -

Relative Risks, Odds Ratios and Cluster Randomised Trials . (1)
1 Relative Risks, Odds Ratios and Cluster Randomised Trials Michael J Campbell, Suzanne Mason, Jon Nicholl Abstract It is well known in cluster randomised trials with a binary outcome and a logistic link that the population average model and cluster specific model estimate different population parameters for the odds ratio. However, it is less well appreciated that for a log link, the population parameters are the same and a log link leads to a relative risk. This suggests that for a prospective cluster randomised trial the relative risk is easier to interpret. Commonly the odds ratio and the relative risk have similar values and are interpreted similarly. However, when the incidence of events is high they can differ quite markedly, and it is unclear which is the better parameter . We explore these issues in a cluster randomised trial, the Paramedic Practitioner Older People’s Support Trial3 . Key words: Cluster randomized trials, odds ratio, relative risk, population average models, random effects models 1 Introduction Given two groups, with probabilities of binary outcomes of π0 and π1 , the Odds Ratio (OR) of outcome in group 1 relative to group 0 is related to Relative Risk (RR) by: . If there are covariates in the model , one has to estimate π0 at some covariate combination. One can see from this relationship that if the relative risk is small, or the probability of outcome π0 is small then the odds ratio and the relative risk will be close. One can also show, using the delta method, that approximately (1). ________________________________________________________________________________ Michael J Campbell, Medical Statistics Group, ScHARR, Regent Court, 30 Regent St, Sheffield S1 4DA [email protected] 2 Note that this result is derived for non-clustered data. -
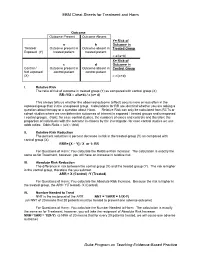
EBM Cheat Sheets for Treatment and Harm Duke Program on Teaching
EBM Cheat Sheets for Treatment and Harm Outcome Outcome Present Outcome Absent Y= Risk of a b Outcome in Treated/ Outcome present in Outcome absent in Treated Group Exposed (Y) treated patient treated patient = a/(a+b) X= Risk of c d Outcome in Control / Outcome present in Outcome absent in Control Group Not exposed control patient control patient (X) = c/(c+d) I. Relative Risk The ratio of risk of outcome in treated group (Y) as compared with control group (X) RR=Y/X = a/(a+b) / c (c+ d) This always tells us whether the observed outcome (effect) occurs more or less often in the exposed group than in the unexposed group. Calculations for RR are identical whether you are asking a question about therapy or a question about Harm. Relative Risk can only be calculated from RCTs or cohort studies where we can determine outcomes of interest in exposed / treated groups and unexposed / control groups. (Note: for case control studies, the numbers of cases and controls and therefore the proportion of individuals with the outcome is chosen by the investigator- for case control studies we use odds ratios: Odds Ratio = (a/c) / (b/d) II. Relative Risk Reduction The percent reduction is percent decrease in risk in the treated group (Y) as compared with control group (X) RRR= [X – Y] / X or 1- RR For Questions of Harm: You calculate the Relative Risk Increase: The calculation is exactly the same as for Treatment, however, you will have an increase in relative risk. III. Absolute Risk Reduction The difference in risk between the control group (X) and the treated group (Y). -

Understanding Relative Risk, Odds Ratio, and Related Terms: As Simple As It Can Get Chittaranjan Andrade, MD
Understanding Relative Risk, Odds Ratio, and Related Terms: As Simple as It Can Get Chittaranjan Andrade, MD Each month in his online Introduction column, Dr Andrade Many research papers present findings as odds ratios (ORs) and considers theoretical and relative risks (RRs) as measures of effect size for categorical outcomes. practical ideas in clinical Whereas these and related terms have been well explained in many psychopharmacology articles,1–5 this article presents a version, with examples, that is meant with a view to update the knowledge and skills to be both simple and practical. Readers may note that the explanations of medical practitioners and examples provided apply mostly to randomized controlled trials who treat patients with (RCTs), cohort studies, and case-control studies. Nevertheless, similar psychiatric conditions. principles operate when these concepts are applied in epidemiologic Department of Psychopharmacology, National Institute research. Whereas the terms may be applied slightly differently in of Mental Health and Neurosciences, Bangalore, India different explanatory texts, the general principles are the same. ([email protected]). ABSTRACT Clinical Situation Risk, and related measures of effect size (for Consider a hypothetical RCT in which 76 depressed patients were categorical outcomes) such as relative risks and randomly assigned to receive either venlafaxine (n = 40) or placebo odds ratios, are frequently presented in research (n = 36) for 8 weeks. During the trial, new-onset sexual dysfunction articles. Not all readers know how these statistics was identified in 8 patients treated with venlafaxine and in 3 patients are derived and interpreted, nor are all readers treated with placebo. These results are presented in Table 1. -

Outcome Reporting Bias in COVID-19 Mrna Vaccine Clinical Trials
medicina Perspective Outcome Reporting Bias in COVID-19 mRNA Vaccine Clinical Trials Ronald B. Brown School of Public Health and Health Systems, University of Waterloo, Waterloo, ON N2L3G1, Canada; [email protected] Abstract: Relative risk reduction and absolute risk reduction measures in the evaluation of clinical trial data are poorly understood by health professionals and the public. The absence of reported absolute risk reduction in COVID-19 vaccine clinical trials can lead to outcome reporting bias that affects the interpretation of vaccine efficacy. The present article uses clinical epidemiologic tools to critically appraise reports of efficacy in Pfzier/BioNTech and Moderna COVID-19 mRNA vaccine clinical trials. Based on data reported by the manufacturer for Pfzier/BioNTech vaccine BNT162b2, this critical appraisal shows: relative risk reduction, 95.1%; 95% CI, 90.0% to 97.6%; p = 0.016; absolute risk reduction, 0.7%; 95% CI, 0.59% to 0.83%; p < 0.000. For the Moderna vaccine mRNA-1273, the appraisal shows: relative risk reduction, 94.1%; 95% CI, 89.1% to 96.8%; p = 0.004; absolute risk reduction, 1.1%; 95% CI, 0.97% to 1.32%; p < 0.000. Unreported absolute risk reduction measures of 0.7% and 1.1% for the Pfzier/BioNTech and Moderna vaccines, respectively, are very much lower than the reported relative risk reduction measures. Reporting absolute risk reduction measures is essential to prevent outcome reporting bias in evaluation of COVID-19 vaccine efficacy. Keywords: mRNA vaccine; COVID-19 vaccine; vaccine efficacy; relative risk reduction; absolute risk reduction; number needed to vaccinate; outcome reporting bias; clinical epidemiology; critical appraisal; evidence-based medicine Citation: Brown, R.B. -

NUMBER NEEDED to TREAT (CONTINUATION) Confidence
Number Needed to treat Confidence Interval for NNT • As with other estimates, it is important that the uncertainty in the estimated number needed to treat is accompanied by a confidence interval. • A common method is that proposed by Cook and Sacket (1995) and based on calculating first the confidence interval NUMBER NEEDED TO TREAT for the difference between the two proportions. • The result of this calculation is 'inverted' (ie: 1/CI) to give a (CONTINUATION) confidence interval for the NNT. Hamisu Salihu, MD, PhD Confidence Interval for NNT Confidence Interval for NNT • This computation assumes that ARR estimates from a sample of similar trials are normally distributed, so we can use the 95th percentile point from the standard normal distribution, z0.95 = 1.96, to identify the upper and lower boundaries of the 95% CI. Solution Class Exercise • In a parallel clinical trial to test the benefit of additional anti‐ hyperlipidemic agent to standard protocol, patients with acute Myocardial Infarction (MI) were randomly assigned to the standard therapy or the new treatment that contains standard therapy and simvastatin (an HMG‐CoA reductase inhibitor). At the end of the study, of the 2223 patients in the control group 622 died as compared to 431 of 2221 patients in the Simvastatin group. – Calculate the NNT and the 95% CI for Simvastatin – Is Simvastatin beneficial as additional therapy in acute cases of MI? 1 Confidence Interval Number Needed to for NNT Treat • The NNT to prevent one additional death is 12 (95% • A negative NNT indicates that the treatment has a CI, 9‐16). -

Making Comparisons
Making comparisons • Previous sessions looked at how to describe a single group of subjects • However, we are often interested in comparing two groups Data can be interpreted using the following fundamental questions: • Is there a difference? Examine the effect size • How big is it? • What are the implications of conducting the study on a sample of people (confidence interval) • Is the effect real? Could the observed effect size be a chance finding in this particular study? (p-values or statistical significance) • Are the results clinically important? 1 Effect size • A single quantitative summary measure used to interpret research data, and communicate the results more easily • It is obtained by comparing an outcome measure between two or more groups of people (or other object) • Types of effect sizes, and how they are analysed, depend on the type of outcome measure used: – Counting people (i.e. categorical data) – Taking measurements on people (i.e. continuous data) – Time-to-event data Example Aim: • Is Ventolin effective in treating asthma? Design: • Randomised clinical trial • 100 micrograms vs placebo, both delivered by an inhaler Outcome measures: • Whether patients had a severe exacerbation or not • Number of episode-free days per patient (defined as days with no symptoms and no use of rescue medication during one year) 2 Main results proportion of patients Mean No. of episode- No. of Treatment group with severe free days during the patients exacerbation year GROUP A 210 0.30 (63/210) 187 Ventolin GROUP B placebo 213 0.40 (85/213) -

Relative Risk Cutoffs For-Statistical-Significance
V0b Relative Risk Cutoffs for Statistical Significance 2014 Milo Schield, Augsburg College Abstract: Relative risks are often presented in the everyday media but are seldom mentioned in introductory statistics courses or textbooks. This paper reviews the confidence interval associated with a relative risk ratio. Statistical significance is taken to be any relative risk where the associated 90% confidence interval does not include unity. The exact solution for the minimum statistically-significant relative risk is complex. A simple iterative solution is presented. Several simplifications are examined. A Poisson solution is presented for those times when the Normal is not justified. 1. Relative risk in the everyday media In the everyday media, relative risks are presented in two ways: explicitly using the phrase “relative risk” or implicitly involving an explicit comparison of two rates or percentages, or by simply presenting two rates or percentages from which a comparison or relative risk can be easily generated. Here are some examples (Burnham, 2009): the risk of developing acute non-lymphoblastic leukaemia was seven times greater compared with children who lived in the same area , but not next to a petrol station Bosch and colleagues ( p 699 ) report that the relative risk of any stroke was reduced by 32 % in patients receiving ramipril compared with placebo Women of normal weight and who were physically active but had high glycaemic loads and high fructose intakes were also at greater risk (53 % and 57 % increase respectively ) than -

HAR202: Introduction to Quantitative Research Skills
HAR202: Introduction to Quantitative Research Skills Dr Jenny Freeman [email protected] Page 1 Contents Course Outline: Introduction to Quantitative Research Skills 3 Timetable 5 LECTURE HANDOUTS 7 Introduction to study design 7 Data display and summary 18 Sampling with confidence 28 Estimation and hypothesis testing 36 Living with risk 43 Categorical data 49 Simple tests for continuous data handout 58 Correlation and Regression 69 Appendix 79 Introduction to SPSS for Windows 79 Displaying and tabulating data lecture handout 116 Useful websites*: 133 Glossary of Terms 135 Figure 1: Statistical methods for comparing two independent groups or samples 143 Figure 2: Statistical methods for differences or paired samples 144 Table 1: Statistical methods for two variables measured on the same sample of subjects 145 BMJ Papers 146 Sifting the evidence – what’s wrong with significance tests? 146 Users’ guide to detecting misleading claims in clinical research papers 155 Scope tutorials 160 The visual display of quantitative information 160 Describing and summarising data 165 The Normal Distribution 169 Hypothesis testing and estimation 173 Randomisation in clinical investigations 177 Basic tests for continuous Normally distributed data 181 Mann-Whitney U and Wilcoxon Signed Rank Sum tests 184 The analysis of categorical data 188 Fisher’s Exact test 192 Use of Statistical Tables 194 Exercises and solutions 197 Displaying and summarising data 197 Sampling with confidence 205 Estimation and hypothesis testing 210 Risk 216 Correlation and Regression 223 Page 2 Course Outline: Introduction to Quantitative Research Skills This module will introduce students to the basic concepts and techniques in quantitative research methods. -

Measures of Effect & Potential Impact
Measures of Effect & Potential Impact Madhukar Pai, MD, PhD Assistant Professor McGill University, Montreal, Canada [email protected] 1 Big Picture Measures of Measures of Measures of potential disease freq effect impact First, we calculate measure of disease frequency in Group 1 vs. Group 2 (e.g. exposed vs. unexposed; treatment vs. placebo, etc.) Then we calculate measures of effect (which aims to quantify the strength of the association): Either as a ratio or as a difference: Ratio = (Measure of disease, group 1) / (Measure of disease, group 2) Difference = (Measure of disease, group 1) - (Measure of disease, group 2) Lastly, we calculate measures of impact, to address the question: if we removed or reduced the exposure, then how much of the disease burden can we reduce? Impact of exposure removal in the exposed group (AR, AR%) Impact of exposure remove in the entire population (PAR, PAR%) 2 Note: there is some overlap between measures of effect and impact 3 Measures of effect: standard 2 x 2 contingency epi table for count data (cohort or case-control or RCT or cross-sectional) Disease - Disease - no Column yes total (Margins) Exposure - a b a+b yes Exposure - c d c+d no Row total a+c b+d a+b+c+d (Margins) 4 Measures of effect: 2 x 2 contingency table for a cohort study or RCT with person-time data Disease - Disease - Total yes no Exposure - a - PYe yes Exposure – c - PY0 no Total a+c - PYe + PY0 Person-time in the unexposed group = PY0 5 Person-time in the exposed group = PYe STATA format for a 2x2 table [note that exposure