DPP-4 Inhibitors
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Does Addition of Vildagliptin to Metformin Monotherapy Improve Glycemic Control in Patients with Type 2 Diabetes Mellitus?
PRACTICE pOINT www.nature.com/clinicalpractice/endmet Does addition of vildagliptin to metformin monotherapy improve glycemic control in patients with type 2 diabetes mellitus? Original article Bosi E et al. (2007) Effects of vildagliptin on OUTCOME MEASURES glucose control over 24 weeks in patients with type 2 diabetes The primary outcome measure was the change inadequately controlled with metformin. Diabetes Care 30: 890–895 in HbA1c level from baseline to study end. Secondary outcome measures included FPG SYNOPSIS levels, lipid profiles, β-cell function, and the KEYWORDS dipeptidyl peptidase 4 inhibitor, incidence and severity of adverse events. glycemic control, HbA1c, type 2 diabetes mellitus, vildagliptin RESULTS BACKGROUND The 50 mg vildagliptin, 100 mg vildagliptin Metformin is frequently prescribed as the and placebo groups comprised 177, 185, and first-line treatment for type 2 diabetes mellitus 182 patients, respectively. Participants were (T2DM); however, additional antidiabetic agents predominantly white and obese, with a mean might be required when glycemic control is age of 54 years. The mean duration of T2DM poor. was 6.2 years, the mean duration of metformin monotherapy was 17 months, and the mean OBJECTIVE metformin dose was 2,100 mg daily. The study To assess the efficacy and safety of the dipeptidyl was completed by >83% of patients in each peptidase 4 (DPP4) inhibitor vildagliptin as group. The mean baseline HbA1c level was 8.4% an add-on to metformin monotherapy. and the mean baseline FPG level was 9.9 mmol/l. Treatment with vildagliptin improved both of DESIGN AND INTERVENTION these parameters. The between-treatment This was a 24-week, multicenter, double- difference (vildagliptin minus placebo) in HbA1c blind, placebo-controlled, randomized study at study end was –0.7% and –1.1% with 50 mg of patients with T2DM inadequately controlled and 100 mg vildagliptin, respectively (P <0.001 with metformin monotherapy. -

Galvus Data Sheet
1 NEW ZEALAND DATA SHEET 1 GALVUS (50 mg tablets) 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Vildagliptin: 1-[(3-Hydroxy-adamant-1-ylamino)-acetyl]-pyrrolidine-2(S)-carbonitrile. One tablet of Galvus® contains 50 mg of vildagliptin. For the full list of excipients, see section 6.1. 3 PHARMACEUTICAL FORM Galvus 50 mg: white to light yellowish, round (8 mm diameter) flat faced with beveled edges, unscored tablet. One side is debossed with "NVR", and the other side with "FB". 4 CLINICAL PARTICULARS 4.1 Therapeutic indications Galvus is indicated as an adjunct to diet and exercise to improve glycaemic control in patients with type 2 diabetes mellitus. • as monotherapy. • in dual combination with metformin, a sulphonylurea (SU), or a thiazolidinedione (TZD) when diet, exercise and a single antidiabetic agent do not result in adequate glycaemic control. • In triple combination with a sulphonylurea and metformin when diet and exercise plus dual therapy with these agents do not provide adequate glycaemic control. • In combination with insulin (with or without metformin) when diet, exercise and a stable dose of insulin do not result in adequate glycaemic control. 4.2 Dosage and method of administration Dose The management of antidiabetic therapy should be individualized. The recommended dose of Galvus is 50 mg once or twice daily. The maximum daily dose of Galvus is 100 mg. For monotherapy, and for combination with metformin, with a TZD or with insulin (with or without metformin), the recommended dose of Galvus is 50 mg or 100 mg daily. When used in dual combination with a sulphonylurea, the recommended dose of vildagliptin is 50 mg once daily. -

Vildagliptin/Metformin Hydrochloride Novartis, INN-Vildagliptin/Metformin
European Medicines Agency Evaluation of Medicines for Human Use Doc.Ref.: EMEA/CHMP/656453/2008 ASSESSMENT REPORT FOR Vildagliptin/metformin hydrochloride Novartis International Nonproprietary Name: vildagliptin / metformin hydrochloride Procedure No. EMEA/H/C/001050 Assessment Report as adopted by the CHMP with all information of a commercially confidential nature deleted. 7 Westferry Circus, Canary Wharf, London, E14 4HB, UK Tel. (44-20) 74 18 84 00 Fax (44-20) 74 18 84 16 E-mail: [email protected] http://www.emea.europa.eu © European Medicines Agency, 2008. Reproduction is authorised provided the source is acknowledged TABLE OF CONTENTS 1. BACKGROUND INFORMATION ON THE PROCEDURE........................................... 3 1.1 Submission of the dossier ........................................................................................................ 3 1.2 Steps taken for the assessment of the product.......................................................................... 3 2. SCIENTIFIC DISCUSSION................................................................................................. 4 2.1 Introduction.............................................................................................................................. 4 2.2 Quality aspects......................................................................................................................... 4 2.3 Non-clinical aspects................................................................................................................. 4 2.4 Clinical -
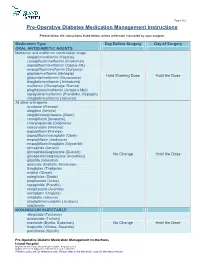
Pre-Operative Diabetes Medication Management Instructions
Page 1 of 2 Pre-Operative Diabetes Medication Management Instructions Please follow the instructions listed below unless otherwise instructed by your surgeon Medication Type Day Before Surgery Day of Surgery ORAL ANTIDIABETIC AGENTS Metformin and metformin combination drugs alogliptin/metformin (Kazano) canagliflozin/metformin (Invokamet) dapagliflozin/metformin (Xigduo XR) empagliflozin/metformin (Synjardy) glipizide/metformin (Metaglip) Hold Evening Dose Hold the Dose glyburide/metformin (Glucovance) linagliptin/metformin (Jentadueto) metformin (Glucophage, Riomet) pioglitazone/metformin (Actoplus Met) repaglidine/metformin (PrandiMet, Repaglin) sitagliptin/metformin (Janumet) All other oral agents acarbose (Precose) alogliptin (Nesina) alogliptin/pioglitazone (Oseni) canagliflozin (Invokana) chlorpropamide (Diabinese) colesevelam (Welchol) dapagliflozin (Farxiga) dapagliflozin/saxagliptin (Qtern) empagliflozin (Jardiance) empagliflozin/linagliptin (Glyxambi) glimepiride (Amaryl) glimepiride/pioglitazone (Duetact) No Change Hold the Dose glimepiride/rosiglitazone (Avandaryl) glipizide (Glucotrol) glyburide (DiaBeta, Micronase) linagliptan (Tradjenta) miglitol (Glyset) nateglinide (Starlix) pioglitazone (Actos) repaglinide (Prandin) rosiglitazone (Avandia) saxagliptin (Onglyza) sitagliptin (Januvia) sitagliptin/simvastatin (Juvisync) tolazamide NON-INSULIN INJECTABLE albiglutide (Tanzeum) dulaglutide (Trulicity) exenatide (Byetta, Bydureon) No Change Hold the Dose liraglutide (Victoza, Saxenda) pramlintide (Symlin) Pre-Operative Diabetic -

Alzheimer's Disease Prevention: from Risk Factors to Early Intervention
Crous-Bou et al. Alzheimer's Research & Therapy (2017) 9:71 DOI 10.1186/s13195-017-0297-z REVIEW Open Access Alzheimer’s disease prevention: from risk factors to early intervention Marta Crous-Bou1, Carolina Minguillón1, Nina Gramunt1,2 and José Luis Molinuevo1,2* Abstract Due to the progressive aging of the population, Alzheimer’s disease (AD) is becoming a healthcare burden of epidemic proportions for which there is currently no cure. Disappointing results from clinical trials performed in mild–moderate AD dementia combined with clear epidemiological evidence on AD risk factors are contributing to the development of primary prevention initiatives. In addition, the characterization of the long asymptomatic stage of AD is allowing the development of intervention studies and secondary prevention programmes on asymptomatic at-risk individuals, before substantial irreversible neuronal dysfunction and loss have occurred, an approach that emerges as highly relevant. In this manuscript, we review current strategies for AD prevention, from primary prevention strategies based on identifying risk factors and risk reduction, to secondary prevention initiatives based on the early detection of the pathophysiological hallmarks and intervention at the preclinical stage of the disease. Firstly, we summarize the evidence on several AD risk factors, which are the rationale for the establishment of primary prevention programmes as well as revising current primary prevention strategies. Secondly, we review the development of public–private partnerships for disease prevention that aim to characterize the AD continuum as well as serving as platforms for secondary prevention trials. Finally, we summarize currently ongoing clinical trials recruiting participants with preclinical AD or a higher risk for the onset of AD-related cognitive impairment. -

OSENI (Alogliptin and Pioglitazone) Tablets II May Increase Risk
HIGHLIGHTS OF PRESCRIBING INFORMATION -----------------------WARNINGS AND PRECAUTIONS--------------------- These highlights do not include all the information needed to use • Congestive heart failure: Fluid retention may occur and can OSENI safely and effectively. See full prescribing information for exacerbate or lead to congestive heart failure. Combination use OSENI. with insulin and use in congestive heart failure NYHA Class I and OSENI (alogliptin and pioglitazone) tablets II may increase risk. Monitor patients for signs and symptoms. Initial U.S. Approval: 2013 (5.1) • Acute pancreatitis: There have been postmarketing reports of WARNING: CONGESTIVE HEART FAILURE acute pancreatitis. If pancreatitis is suspected, promptly See full prescribing information for complete boxed warning discontinue OSENI. (5.2) • Thiazolidinediones, including pioglitazone, cause or • Hypersensitivity: There have been postmarketing reports of exacerbate congestive heart failure in some patients. (5.1) serious hypersensitivity reactions in patients treated with alogliptin • After initiation of OSENI and after dose increases, monitor such as anaphylaxis, angioedema and severe cutaneous adverse patients carefully for signs and symptoms of heart failure reactions. In such cases, promptly discontinue OSENI, assess for (e.g., excessive, rapid weight gain, dyspnea and/or other potential causes, institute appropriate monitoring and edema). If heart failure develops, it should be managed treatment and initiate alternative treatment for diabetes. (5.3) according to current standards of care and • Hepatic effects: Postmarketing reports of hepatic failure, discontinuation or dose reduction of pioglitazone in OSENI sometimes fatal. Causality cannot be excluded. If liver injury is must be considered. detected, promptly interrupt OSENI and assess patient for • OSENI is not recommended in patients with symptomatic probable cause, then treat cause if possible, to resolution or heart failure. -

Dipeptidyl Peptidase-4 Inhibitor Switching
abetes & Di M f e t o a Tanaka et al., J Diabetes Metab 2016, 7:9 l b a o n l r i DOI: 10.4172/2155-6156.1000701 s u m o J Journal of Diabetes & Metabolism ISSN: 2155-6156 Research Article Open Access Dipeptidyl Peptidase-4 Inhibitor Switching as an Alternative Add-on Therapy to Current Strategies Recommended by Guidelines: Analysis of a Retrospective Cohort of Type 2 Diabetic Patients Masami Tanaka*, Takeshi Nishimura, Risa Sekioka and Hiroshi Itoh Department of Internal Medicine, School of Medicine, Keio University 35 Shinanomachi, Shinjuku-ku, Tokyo 160-8582, Japan Abstract Objective: This retrospective cohort study aimed to investigate the significance of dipeptidyl peptidase-4 (DPP- 4) inhibitor switch therapy, which is currently not recommended by major diabetes guidelines. Methods: The subjects were 238 outpatients with type 2 diabetes who had been prescribed sitagliptin 50 mg daily, which was subsequently changed in one of three ways. Patients whose sitagliptin was switched to vildagliptin 50 mg twice daily were defined as the switched group. Patients whose sitagliptin was increased to 100 mg once daily were defined as the increased group. Patients who received an additional alfa-glucosidase inhibitor (α-GI) three times daily prior to meals and sitagliptin 50 mg once daily were defined as the added group. The primary endpoint was the glycated hemoglobin (HbA1c) value at 6 months after the medication change. Patients whose oral hypoglycemic agents were changed within 6 months after switching to vildagliptin, increasing sitagliptin, or adding α-GI were excluded from the full analysis set and the remaining patients were included in the per protocol analysis. -

The Role of Statins in Both Cognitive Impairment and Protection Against Dementia: a Tale of Two Mechanisms Bob G
Schultz et al. Translational Neurodegeneration (2018) 7:5 https://doi.org/10.1186/s40035-018-0110-3 REVIEW Open Access The role of statins in both cognitive impairment and protection against dementia: a tale of two mechanisms Bob G. Schultz, Denise K. Patten and Daniel J. Berlau* Abstract Nearly 30% of adults 40 years and older in the United States are on a statin. Their widespread use heightens the importance of careful consideration of their varied effects on the body. Although randomized controlled trials have not confirmed cognitive impairing effects with statins, continuing evidence suggests statins have the ability to cause reversible cognitive impairment in some patients. Paradoxically, statins have also been shown to decrease the risk of dementia, Alzheimer’s disease, and improve cognitive impairment in some cases. However, randomized controlled trials have similarly failed to find the beneficial effect. Supporting evidence for both claims is compelling whereas known limitations of the clinical trials may explain the lack of findings. This narrative review aims to explain why there is still controversy and how both effects can, and may, be possible. The mechanisms that have been hypothesized for each effect are seemingly independent from one another and may explain the contradicting results. Being mindful of the complex effects of statins, health care providers need to be able to identify patients who are at risk for or already experiencing cognitive impairment from statin use while also identifying those who could potentially decrease their risk of dementia with statins. Keywords: Statin, Cognition, Dementia, Memory, Cholesterol, Alzheimer’s disease, Vascular dementia, Neuroprotection Background people in the United States (10% of Americans) are cur- Cardiovascular disease (CVD) is the leading cause of rently on a statin with an estimated 56 million people morbidity and mortality in the United States and ele- (24% of Americans) eligible to consider a statin. -

Primary and Secondary Prevention Interventions for Cognitive Decline
2016 Primary and secondary prevention interventions for cognitive decline and dementia Overview of reviews Published by The Norwegian Institute of Public Health Section for evidence summaries in the Knowledge Centre Title Primary and secondary prevention interventions for cognitive decline and dementia Norwegian title Primær‐ og sekundærforebyggende tiltak for kognitiv svikt og demens Responsible Camilla Stoltenberg, direktør Authors Gerd M Flodgren, project leader, researcher, the Knowledge Centre Rigmor C Berg, Head of Unit, for Social Welfare Research at the Knowledge Centre ISBN 978‐82‐8082‐745‐6 Projectnumber 798 Type of publication Overview of reviews No of pages 69 (110 inklusiv vedlegg) Client Nasjonalforeningen for folkehelsen MeSH terms Alzheimer’s disease, dementia, cognition, cognitive impairment, cognitive disorders, memory complaints, primary prevention, secondary prevention Citation Flodgren GM, Berg RC. Primary and secondary prevention interventions for cognitive decline and dementia. [Primær‐ og sekundærforebyggende tiltak for kognitiv svikt og demens] Rapport −2016. Oslo: Folkehelseinstituttet, 2016. 2 Table of contents Table of contents TABLE OF CONTENTS 3 KEY MESSAGES 5 EXECUTIVE SUMMARY 6 Background 6 Objectives 6 Methods 6 Results 6 Discussion 8 Conclusions 8 HOVEDFUNN (NORSK) 9 SAMMENDRAG (NORSK) 10 Bakgrunn 10 Problemstillinger 10 Metoder 10 Resultat 10 Diskusjon 12 Konklusjon 12 PREFACE 13 OBJECTIVES 15 BACKGROUND 16 Description of the condition 16 How the interventions may work 18 Why is it important to do this -

Eucreas, INN-Vildagliptin / Metformin Hydrochloride
SCIENTIFIC DISCUSSION 1. Introduction The pathophysiology of Type 2 diabetes mellitus (T2DM) is characterised by deficient insulin activity arising from decreased insulin secretion secondary to beta cell failure, and/or compromised insulin action in peripheral target tissues (insulin resistance). This abnormal metabolic state is exacerbated by excess hepatic glucose production and altered metabolism of proteins and lipids, which along with hyperglycaemia, contribute to microvascular and macrovascular complications. T2DM accounts for approximately 85% to 95% of diabetes cases in developed regions like the European Union. Age and weight are established risk factors for T2DM. The majority of patients with T2DM are overweight or obese. Diet modification and exercise is the first line of treatment for T2DM. Pharmacologic intervention with one oral antidiabetic drug (OAD) is usually the next step in treatment. After 3 to 9 years of OAD monotherapy, patients typically require an additional intervention. The recommended first line treatment is metformin, which restrains hepatic glucose production and decreases peripheral insulin resistance. Sulphonylureas, which are insulin secretagogues, may be used as an alternative to patients intolerant to metformin, or as an addition to metformin. Other second line oral treatment alternatives include alpha-glucosidase inhibitors, meglitinides and thiazolidinediones. Recently the first GLP-1 analogue, exenatide, and the first DPP-4 inhibitors, sitagliptin and vildagliptin, were approved by the CHMP. Vildagliptin belongs to a new class of oral anti-diabetic drugs and is a selective and reversible inhibitor of Dipeptidyl peptidase 4 (DPP-4), the enzyme which inactivates the incretin hormones, glucagon-like peptide-1 (GLP-1), and glucose-dependent insulinotropic polypeptide (GIP), hormones which significantly contribute to the maintenance of glucose homeostasis. -

Can the Treatment of Hypertension in the Middle-Aged Prevent Dementia in the Elderly?
High Blood Press Cardiovasc Prev DOI 10.1007/s40292-016-0144-5 REVIEW ARTICLE Can the Treatment of Hypertension in the Middle-Aged Prevent Dementia in the Elderly? 1 1 1 1 Antonio Coca • Eila Monteagudo • Mo´nica Dome´nech • Miguel Camafort • Cristina Sierra1 Received: 16 February 2016 / Accepted: 28 March 2016 Ó Springer International Publishing Switzerland 2016 Abstract Hypertension, one of the main risk factors for 1 Introduction cardiovascular disease, is thought to play a crucial role in the pathophysiology of cognitive impairment. Studies have Cardiovascular disease (CVD) remains the leading cause of associated hypertension with subjective cognitive failures death worldwide. The Global Burden of Disease study and objective cognitive decline. Subjective cognitive fail- estimated that 29.6 % of all deaths were caused by CVD in ures may reflect the early phase of a long pathological 2010, more than all communicable, maternal, neonatal and process leading to cognitive decline and dementia that has nutritional disorders combined, and twice the number of been associated with hypertension and other cardiovascular deaths caused by cancer [1]. The burden of CVD in Europe risk factors. The underlying cerebral structural change remains heavy, although the prevalence varies dramatically associated with cognitive decline may be a consequence of among countries. More than four million Europeans die of the cerebral small-vessel disease induced by high blood CVD every year, and many more are hospitalized after pressure and may be detected on magnetic resonance acute episodes or are treated for chronic CVD [2]. The imaging as white matter hyperintensities, cerebral percentage of the population affected by hypertension, one microbleeds, lacunar infarcts or enlarged perivascular of the main risk factors for CVD, is remarkably high and, spaces. -

NESINA (Alogliptin) Tablets, for Oral Use • Heart Failure: Consider the Risks and Benefits of NESINA Prior to Initial U.S
HIGHLIGHTS OF PRESCRIBING INFORMATION ------------------------WARNINGS AND PRECAUTIONS---------------------- These highlights do not include all the information needed to use • Acute pancreatitis: There have been postmarketing reports of NESINA safely and effectively. See full prescribing information for acute pancreatitis. If pancreatitis is suspected, promptly NESINA. discontinue NESINA. (5.1) NESINA (alogliptin) tablets, for oral use • Heart failure: Consider the risks and benefits of NESINA prior to Initial U.S. Approval: 2013 initiating treatment in patients at risk for heart failure. If heart failure develops, evaluate and manage according to current ---------------------------RECENT MAJOR CHANGES-------------------------- standards of care and consider discontinuation of NESINA (5.2). Indications and Usage (1.1) 4/2016 • Hypersensitivity: There have been postmarketing reports of Dosage and Administration serious hypersensitivity reactions in patients treated with NESINA Patients with Renal Impairment (2.2) 4/2016 such as anaphylaxis, angioedema and severe cutaneous adverse Warnings and Precautions reactions, including Stevens-Johnson syndrome. In such cases, Pancreatitis (5.1) 4/2016 promptly discontinue NESINA, assess for other potential causes, Heart Failure (5.2) 4/2016 institute appropriate monitoring and treatment and initiate Hepatic Effects (5.4) 4/2016 alternative treatment for diabetes. (5.3) Bullous Pemphigoid (5.7) 12/2016 • Hepatic effects: Postmarketing reports of hepatic failure, ----------------------------INDICATIONS AND USAGE--------------------------- sometimes fatal. Causality cannot be excluded. If liver injury is NESINA is a dipeptidyl peptidase-4 (DPP-4) inhibitor indicated as an detected, promptly interrupt NESINA and assess patient for adjunct to diet and exercise to improve glycemic control in adults with probable cause, then treat cause if possible, to resolution or type 2 diabetes mellitus.