Structural and Functional Differentiation of Bacterial Communities
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Multilayered Horizontal Operon Transfers from Bacteria Reconstruct a Thiamine Salvage Pathway in Yeasts
Multilayered horizontal operon transfers from bacteria reconstruct a thiamine salvage pathway in yeasts Carla Gonçalvesa and Paula Gonçalvesa,1 aApplied Molecular Biosciences Unit-UCIBIO, Departamento de Ciências da Vida, Faculdade de Ciências e Tecnologia, Universidade Nova de Lisboa, 2829-516 Caparica, Portugal Edited by Edward F. DeLong, University of Hawaii at Manoa, Honolulu, HI, and approved September 22, 2019 (received for review June 14, 2019) Horizontal acquisition of bacterial genes is presently recognized as nisms presumed to have facilitated a transition from bacterial an important contribution to the adaptation and evolution of operon transcription to eukaryotic-style gene expression were eukaryotic genomes. However, the mechanisms underlying ex- proposed, such as gene fusion giving rise to multifunctional pro- pression and consequent selection and fixation of the prokaryotic teins (6, 23, 24), increase in intergenic distances between genes to genes in the new eukaryotic setting are largely unknown. Here we generate room for eukaryotic promoters, and independent tran- show that genes composing the pathway for the synthesis of the scription producing mRNAs with poly(A) tails have been dem- essential vitamin B1 (thiamine) were lost in an ancestor of a yeast onstrated (22). In the best documented study, which concerns a lineage, the Wickerhamiella/Starmerella (W/S) clade, known to bacterial siderophore biosynthesis operon acquired by yeasts be- harbor an unusually large number of genes of alien origin. The longing to the Wickerhamiella/Starmerella (W/S) clade, the bacte- thiamine pathway was subsequently reassembled, at least twice, rial genes acquired as an operon were shown to be functional (22). by multiple HGT events from different bacterial donors involving Thiamine, commonly known as vitamin B1, is essential for all both single genes and entire operons. -

Short-Interval Reburns in the Boreal Forest Alter Soil Bacterial Communities, Reflecting Increased Ph and Poor Conifer Seedling
bioRxiv preprint doi: https://doi.org/10.1101/2021.03.31.437944; this version posted April 1, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Short-interval reburns in the boreal forest alter soil bacterial communities, reflecting 2 increased pH and poor conifer seedling establishment 3 4 Jamie Wooleta,b, Ellen Whitmanc,d, Marc-André Parisienc, Dan K. Thompsonc, Mike D. 5 Flannigand, and Thea Whitmana* 6 a. Department of Soil Science, University of Wisconsin-Madison, 1525 Observatory Dr., 7 Madison, WI, 53706, USA 8 b. Department of Forest and Rangeland Stewardship, Colorado State University, 1001 Amy Van 9 Dyken Way, Fort Collins, CO, 80521, USA 10 c. Northern Forestry Centre, Canadian Forest Service, Natural Resources Canada, 5320 122 11 Street, Edmonton, AB, T6H 3S5, Canada 12 d. Department of Renewable Resources, University of Alberta, 751 General Services Building, 13 Edmonton, AB, T6G 2H1, Canada 14 * Corresponding author: [email protected]; 608.263.4947 15 16 Abstract: Increasing burn rates (percentage area burned annually) in some biomes are leading to 17 fires burning in close succession, triggering rapid vegetation change as well as altering soil 18 properties. Despite the importance of soil microbes for nutrient cycling and as plant symbionts, 19 the effects of increased fire frequency on belowground microbial communities remain largely 20 unknown. We present a study of the effects of short interval reburns (defined here as <20 years 21 between fires) on soil bacterial communities in the boreal forest of northwestern Canada, using a 22 paired site design that spans wetlands and uplands, with 50 sites total. -

Inoculation with Mycorrhizal Fungi and Irrigation Management Shape the Bacterial and Fungal Communities and Networks in Vineyard Soils
microorganisms Article Inoculation with Mycorrhizal Fungi and Irrigation Management Shape the Bacterial and Fungal Communities and Networks in Vineyard Soils Nazareth Torres † , Runze Yu and S. Kaan Kurtural * Department of Viticulture and Enology, University of California Davis, 1 Shields Avenue, Davis, CA 95616, USA; [email protected] (N.T.); [email protected] (R.Y.) * Correspondence: [email protected] † Current address: Advanced Fruit and Grape Growing Group, Public University of Navarra, 31006 Pamplona, Spain. Abstract: Vineyard-living microbiota affect grapevine health and adaptation to changing environ- ments and determine the biological quality of soils that strongly influence wine quality. However, their abundance and interactions may be affected by vineyard management. The present study was conducted to assess whether the vineyard soil microbiome was altered by the use of biostimulants (arbuscular mycorrhizal fungi (AMF) inoculation vs. non-inoculated) and/or irrigation management (fully irrigated vs. half irrigated). Bacterial and fungal communities in vineyard soils were shaped by both time course and soil management (i.e., the use of biostimulants and irrigation). Regarding alpha diversity, fungal communities were more responsive to treatments, whereas changes in beta diversity were mainly recorded in the bacterial communities. Edaphic factors rarely influence bacte- rial and fungal communities. Microbial network analyses suggested that the bacterial associations Citation: Torres, N.; Yu, R.; Kurtural, were weaker than the fungal ones under half irrigation and that the inoculation with AMF led to S.K. Inoculation with Mycorrhizal the increase in positive associations between vineyard-soil-living microbes. Altogether, the results Fungi and Irrigation Management highlight the need for more studies on the effect of management practices, especially the addition Shape the Bacterial and Fungal of AMF on cropping systems, to fully understand the factors that drive their variability, strengthen Communities and Networks in Vineyard Soils. -

Effect of Vertical Flow Exchange on Microbial Community Dis- Tributions in Hyporheic Zones
Article 1 by Heejung Kim and Kang-Kun Lee* Effect of vertical flow exchange on microbial community dis- tributions in hyporheic zones School of Earth and Environmental Sciences, Seoul National University, Seoul 08826, Republic of Korea; *Corresponding author, E-mail: [email protected] (Received: November 2, 2018; Revised accepted: January 6, 2019) https://doi.org/10.18814/epiiugs/2019/019001 The effect of the vertical flow direction of hyporheic flux advance of hydrodynamic modeling has improved research of hydro- on the bacterial community is examined. Vertical velocity logical exchange processes at the hyporheic zone (Cardenas and Wil- change of the hyporheic zone was examined by installing son, 2007; Fleckenstein et al., 2010; Endreny et al., 2011). Also, this a piezometer on the site, and a total of 20,242 reads were zone has plentiful micro-organisms. The hyporheic zone constituents analyzed using a pyrosequencing assay to investigate the a dynamic hotspot (ecotone) where groundwater and surface water diversity of bacterial communities. Proteobacteria (55.1%) mix (Smith et al., 2008). were dominant in the hyporheic zone, and Bacteroidetes This area constitutes a flow path along which surface water down wells into the streambed sediment and groundwater up wells in the (16.5%), Actinobacteria (7.1%) and other bacteria phylum stream, travels for some distance before eventually mixing with (Firmicutes, Cyanobacteria, Chloroflexi, Planctomycetesm groundwater returns to the stream channel (Hassan et al., 2015). Sur- and unclassified phylum OD1) were identified. Also, the face water enters the hyporheic zone when the vertical hydraulic head hyporheic zone was divided into 3 points – down welling of surface water is greater than the groundwater (down welling). -

Table S5. the Information of the Bacteria Annotated in the Soil Community at Species Level
Table S5. The information of the bacteria annotated in the soil community at species level No. Phylum Class Order Family Genus Species The number of contigs Abundance(%) 1 Firmicutes Bacilli Bacillales Bacillaceae Bacillus Bacillus cereus 1749 5.145782459 2 Bacteroidetes Cytophagia Cytophagales Hymenobacteraceae Hymenobacter Hymenobacter sedentarius 1538 4.52499338 3 Gemmatimonadetes Gemmatimonadetes Gemmatimonadales Gemmatimonadaceae Gemmatirosa Gemmatirosa kalamazoonesis 1020 3.000970902 4 Proteobacteria Alphaproteobacteria Sphingomonadales Sphingomonadaceae Sphingomonas Sphingomonas indica 797 2.344876284 5 Firmicutes Bacilli Lactobacillales Streptococcaceae Lactococcus Lactococcus piscium 542 1.594633558 6 Actinobacteria Thermoleophilia Solirubrobacterales Conexibacteraceae Conexibacter Conexibacter woesei 471 1.385742446 7 Proteobacteria Alphaproteobacteria Sphingomonadales Sphingomonadaceae Sphingomonas Sphingomonas taxi 430 1.265115184 8 Proteobacteria Alphaproteobacteria Sphingomonadales Sphingomonadaceae Sphingomonas Sphingomonas wittichii 388 1.141545794 9 Proteobacteria Alphaproteobacteria Sphingomonadales Sphingomonadaceae Sphingomonas Sphingomonas sp. FARSPH 298 0.876754244 10 Proteobacteria Alphaproteobacteria Sphingomonadales Sphingomonadaceae Sphingomonas Sorangium cellulosum 260 0.764953367 11 Proteobacteria Deltaproteobacteria Myxococcales Polyangiaceae Sorangium Sphingomonas sp. Cra20 260 0.764953367 12 Proteobacteria Alphaproteobacteria Sphingomonadales Sphingomonadaceae Sphingomonas Sphingomonas panacis 252 0.741416341 -

The Potato Yam Phyllosphere Ectosymbiont Paraburkholderia Sp
fmicb-11-00581 April 19, 2020 Time: 8:50 # 1 ORIGINAL RESEARCH published: 21 April 2020 doi: 10.3389/fmicb.2020.00581 The Potato Yam Phyllosphere Ectosymbiont Paraburkholderia sp. Msb3 Is a Potent Growth Promotor in Tomato Johannes B. Herpell1, Florian Schindler1, Mersad Bejtovic´ 1, Lena Fragner1,2, Bocar Diallo1, Anke Bellaire3, Susanne Kublik4, Bärbel U. Foesel4, Silvia Gschwendtner4, Melina Kerou5, Michael Schloter4 and Wolfram Weckwerth1,2* 1 Molecular Systems Biology (MOSYS), Department of Functional and Evolutionary Ecology, University of Vienna, Vienna, Austria, 2 Vienna Metabolomics Center (VIME), University of Vienna, Vienna, Austria, 3 Division of Structural and Functional Botany, Department of Botany and Biodiversity Research, University of Vienna, Vienna, Austria, 4 Research Unit Edited by: for Comparative Microbiome Analysis, German Research Center for Environmental Health, Helmholtz Zentrum München, 5 Michele Perazzolli, Neuherberg, Germany, Archaea Biology and Ecogenomics Division, Department of Functional and Evolutionary Ecology, University of Trento, Italy University of Vienna, Vienna, Austria Reviewed by: Stéphane Compant, The genus Paraburkholderia includes a variety of species with promising features for Austrian Institute of Technology (AIT), sustainable biotechnological solutions in agriculture through increasing crop productivity. Austria Lionel Moulin, Here, we present a novel Paraburkholderia isolate, a permanent and predominant Institut de Recherche Pour le member of the Dioscoreae bulbifera (yam family, Dioscoreaceae) -

Functions, Transmission and Emission of the Canopy Microbiota Tania Fort
Functions, transmission and emission of the canopy microbiota Tania Fort To cite this version: Tania Fort. Functions, transmission and emission of the canopy microbiota. Vegetal Biology. Univer- sité de Bordeaux, 2019. English. NNT : 2019BORD0338. tel-02869590 HAL Id: tel-02869590 https://tel.archives-ouvertes.fr/tel-02869590 Submitted on 16 Jun 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. THÈSE PRESENTÉE POUR OBTENIR LE GRADE DE DOCTEUR DE L’UNIVERSITE DE BORDEAUX ECOLE DOCTORALE SCIENCES ET ENVIRONNEMENTS ECOLOGIE ÉVOLUTIVE, FONCTIONNELLE, ET DES COMMUNAUTÉS Par Tania Fort Fonctions, transmission et émission du microbiote de la canopée Sous la direction de Corinne Vacher Soutenue le 10 décembre 2019 Membres du jury : Mme. Anne-Marie DELORT Directrice de recherche Institut de Chimie de Clermont-Ferrand Rapporteuse M. Stéphane Uroz Directeur de recherche INRA Nancy Rapporteur Mme. Patricia Luis Maître de conférence Université de Lyon 1 Rapporteuse Mme. Annabel Porté Directrice de recherche INRA Bordeaux Présidente Mme. Corinne Vacher Directrice de recherche INRA Bordeaux Directrice Fonctions, transmission et émission du microbiote de la canopée. Les arbres interagissent avec des communautés microbiennes diversifiées qui influencent leur fitness et le fonctionnement des écosystèmes terrestres. -

Isolation of Rhizobacteria in Southwestern Québec, Canada: An
Isolation of rhizobacteria in Southwestern Québec, Canada: An investigation of their impact on the growth and salinity stress alleviation in Arabidopsis thaliana and crop plants Di Fan Department of Plant Science Faculty of Agricultural and Environmental Sciences Macdonald Campus of McGill University 21111 Lakeshore Road, Sainte-Anne-de-Bellevue, Québec H9X 3V9 December 2017 A thesis submitted to McGill University in partial fulfillment of the requirements of the degree of DOCTOR OF PHILOSOPHY © Di Fan, Canada, 2017 Table of contents Abstract ................................................................................................. x Résumé ................................................................................................ xiii Acknowledegments ............................................................................. xv Preface ................................................................................................ xviii Contribution of authors ................................................................................ xviii Chapter 1................................................................................................. 1 Introduction ............................................................................................ 1 Chapter 2................................................................................................. 5 Literature Review ................................................................................... 5 2.1 What are root exudates? ......................................................................... -

LIST of PROKARYOTIC NAMES VALIDLY PUBLISHED in August 2016
Leibniz‐Institut DSMZ‐Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH LIST OF PROKARYOTIC NAMES VALIDLY PUBLISHED in August 2016 compiled by Dorothea Gleim, Leibniz‐Institut DSMZ ‐ Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH Braunschweig, Germany PROKARYOTIC NOMENCLATURE, Update 08/2016 2 Notes This compilation of validly published names of Prokaryotes is produced to the best of our knowledge. Nevertheless we do not accept any responsibility for errors, inaccuracies or omissions. Names of prokaryotes are defined as being validly published by the International Code of Nomenclature of Bacteria a,b. Validly published are all names which are included in the Approved Lists of Bacterial Names c,d,e,f and the names subsequently published in the International Journal of Systematic Bacteriology (IJSB) and, from January 2000, in the International Journal of Systematic and Evolutionary Microbiology (IJSEM) in the form of original articles or in the Validation Lists. Names not considered to be validly published, should no longer be used, or used in quotation marks, i.e. “Streptococcus equisimilis” to denote that the name has no standing in nomenclature. Please note that such names cannot be retrieved in this list. Explanations, Examples Numerical reference followed by Streptomyces setonii 30:401 (AL) Included in The Approved Lists of (AL) Bacterial Names. [Volume:page (AL)] Numerical reference with asterisk Acidiphilium cryptum 31:331* original publication in the IJSB or IJSEM [Volume:page of description*] Numerical reference without Acetomicrobium faecale 38:136 Validation List in the IJSB or IJSEM asterisk [Volume:page] ≡ Acetobacter methanolicus homotypic (formerly: objective) (basonym) ≡ Acidomonas synonym; the original name is methanolica indicated as a basonym a,g = Brevibacterium albidum (as heterotypic (formerly: subjective) synonym) = synonym; the name published Curtobacterium albidum first (Curtobacterium albidum) has priority over Brevibacterium albidum a,g corrig. -

Genome Sequence of Caballeronia Sordidicola Strain PAMC 26577 Isolated from Cladonia Sp., an Arctic Lichen Species
Korean Journal of Microbiology (2017) Vol. 53, No. 2, pp. 141-143 pISSN 0440-2413 DOI https://doi.org/10.7845/kjm.2017.7037 eISSN 2383-9902 Copyright ⓒ 2017, The Microbiological Society of Korea Note (Genome Announcement) Genome sequence of Caballeronia sordidicola strain PAMC 26577 isolated from Cladonia sp., an Arctic lichen species Jhung Ahn Yang1, Soon Gyu Hong2, and Hyun-Myung Oh1* 1Department of Marine-Bio Convergence Science, Specialized Graduate School Science & Technology Convergence, Pukyong National University, Busan 48547, Republic of Korea 2Division of Polar Life Sciences, Korea Polar Research Institute, Incheon 21990, Republic of Korea 북극 지의류 Cladonia 종에서 분리한 Caballeronia sordidicola 균주 PAMC 26577의 유전체 서열 분석 양정안1 ・ 홍순규2 ・ 오현명1* 1부경대학교 과학기술융합전문대학원, 2극지연구소 (Received June 8, 2017; Accepted June 8, 2017) Caballeronia sordidicola strain PAMC 26577 was isolated Cultivable sixty eight isolates from polar lichen species from Cladonia sp., a lichen collected from Svalbard Archipelago were investigated by 16S rRNA phylogenetic analysis and they in the Arctic Ocean. Draft genomic sequences of PAMC 26577 were affiliated with the phyla Actinobacteria, Bacteroidetes, were determined using Illumina and 182 contigs were submitted Deinococcus-Thermus, and Firmicutes and with the classes to GenBank and N50 value was 159,226. The genome of PAMC Alphaproteobacteria, Betaproteobacteria, and Gammaproteo- 26577 was comprised of 8,334,211 base pairs and %G+C bacteria et al. content was 59.4. The genome included 8 ribosomal RNA genes (Lee , 2014). The strain PAMC 26577 was and 51 tRNA genes as non-coding sequences. Protein-coding originally deposited at Polar and Alpine Microbial Collection genes were 8,065 in number and they included central meta- (Lee et al., 2012) and it was reported that the strain PAMC bolism genes as well as butanol/butyrate biosynthesis, poly- 26577 was identified as Burkholderia sordidicola in the class hydroxybutyrate metabolism, serine cycle methylotrophy genes, Betaproteobacteria based on 16S rRNA sequence analysis, and glycogen metabolism. -

Microbial Community Analysis in Soil (Rhizosphere) and the Marine (Plastisphere) Environment in Function of Plant Health and Biofilm Formation
Microbial community analysis in soil (rhizosphere) and the marine (plastisphere) environment in function of plant health and biofilm formation Caroline De Tender Thesis submitted in fulfillment of the requirements for the degree of Doctor (PhD) in Biotechnology Promotors: Prof. Dr. Peter Dawyndt Department of Applied mathematics, Computer Science and Statistics Faculty of Science Ghent University Dr. Martine Maes Crop Protection - Plant Sciences Unit Institute for Agricultural, Fisheries and Food Research (ILVO) Ir. Lisa Devriese Fisheries – Animal Sciences Unit Insitute for Agricultural, Fisheries and Food Research (ILVO) Dank je wel! De allerlaatste woorden die geschreven worden voor deze thesis zijn waarschijnlijk de eerste die gelezen worden door velen. Ongeveer vier jaar geleden startte ik mijn doctoraat bij het ILVO. Met volle moed begon ik aan mijn avontuur. Het ging niet altijd even vlot en ik kan eerlijk bekennen dat meerdere grenzen verlegd zijn. Vooral de combinatie van twee onderwerpen bleek niet altijd evident en kostte me meer dan eens bloed, zweet en tranen. Daarentegen bracht het ook vele opportuniteiten. De enige dag kon ik aan het wroeten zijn in de serre, terwijl ik de dag erop op de Simon Stevin sprong (en dit mag letterlijk worden genomen!) om plastic uit zee te vissen. Ja, het was me wel het avontuur… Natuurlijk zou dit allemaal niet mogelijk geweest zijn zonder de hulp van een aantal geweldige mensen. In de eerste plaats, mijn promotoren: Prof. Peter Dawyndt, dr. Martine Maes en natuurlijk Lisa Devriese. Dank je wel om vier jaar geleden het vertrouwen te hebben om mij dit onderzoek toe te wijzen, me steeds in de juiste richting te duwen als ik het Noorden even kwijt was, maar ook voor de gezellige babbels op de bureau. -
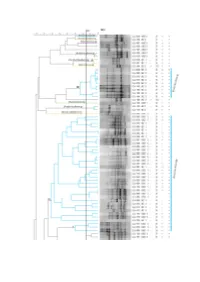
Supplementary File 1 (PDF, 1566 Kib)
Figure S1. Dendrogram based on cluster analysis of fingerprinting PCR profiles of the isolates from A. longifolia nodules, using the Pearson correlation coefficient and the unweighted pair-group method with arithmetic mean algorithm (UPGMA). 84% was the cut-off level below which isolates could be considered different. On the right are represented: isolate identification (CJJ xxx), zone from where it was isolated (UBZ/BZ x), Gram test result, morphology (rods (B) or cocci (CC)), catalase test result and oxidase test result, both (+) or (-). Colours are according to the phylum/class into each genus belong to: Proteobacteria/α-proteobacteria (blue), Proteobacteria/β-proteobacteria (orange), Proteobacteria/γ-proteobacteria (green), firmicutes/Bacilli (purple) and actinobacteria/Actinobacteria (yellow). Roman numbering identifies clusters. Isolates are identified up to genus level. Table S1. Identification of bacterial isolates obtained from unburnt and burnt zones by BLAST analysis of the 16S rRNA gene sequences. GenBank accession numbers are also indicated. GenBank % Pairwise Isolate ID Identification accession Closest hit by BLAST analysis1 Identity numbers CJJ 003 Bradyrhizobium sp. MT465339 Bradyrhizobium sp. MH612954 100% CJJ 008 Bradyrhizobium sp. MT465340 Bradyrhizobium sp. Z94815 100% CJJ 010 Bradyrhizobium sp. MT465341 Bradyrhizobium cytisi MK370569 98.1% CJJ 013 Bradyrhizobium pachyrhizi MT465342 Bradyrhizobium pachyrhizi KP769443 100% CJJ 017 Bradyrhizobium sp. MT465343 Bradyrhizobium cytisi MK370569 99.3% CJJ 020 Bradyrhizobium sp. MT465344 Bradyrhizobium sp. DQ202229 99.5% CJJ 024 Bradyrhizobium cytisi MT465345 Bradyrhizobium cytisi MK30569 100% CJJ 025 Paraburkholderia phytofirmans MT465346 Paraburkholderia phytofirmans NR_102845 100% Bradyrhizobium ganzhouense NR_133706 99.8% CJJ 026 Bradyrhizobium sp. MT465347 Bradyrhizobium rifense NR_116361 99.8% CJJ 027 Althererythrobacter sp. MT465348 Althererythrobacter sp.