JOC Year in Review : 1995 10/14/16
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

James Bond a 50 The
JAMES BOND: SIGNIFYING CHANGING IDENTITY THROUGH THE COLD WAR AND BEYOND By Christina A. Clopton Submitted to Central European University Department of International Relations and European Studies In partial fulfillment of the requirements for the Masters of Arts Supervisor: Professor Alexander Astrov CEU eTD Collection Budapest, Hungary 2014 Word Count: 12,928 Abstract The Constructivist paradigm of International Relations (IR) theory has provided for an ‘aesthetic turn’ in IR. This turn can be applied to popular culture in order to theorize about the international system. Using the case study of the James Bond film series, this paper investigates the continuing relevancy of the espionage series through the Cold War and beyond in order to reveal new information about the nature of the international political system. Using the concept of the ‘empty signifier,’ this work establishes the shifting identity of James Bond in relation to four thematic icons in the films: the villains, locations, women and technology and their relation to the international political setting over the last 50 years of the films. Bond’s changing identity throughout the series reveals an increasingly globalized society that gives prominence to David Chandler’s theory about ‘empire in denial,’ in which Western states are ever more reluctant to take responsibility for their intervention abroad. CEU eTD Collection i Acknowledgements I would like to extend my deepest gratitude to Professor Alexander Astrov for taking a chance with me on this project and guiding me through this difficult process. I would also like to acknowledge the constant support and encouragement from my IRES colleagues through the last year. -

The James Bond Quiz Eye Spy...Which Bond? 1
THE JAMES BOND QUIZ EYE SPY...WHICH BOND? 1. 3. 2. 4. EYE SPY...WHICH BOND? 5. 6. WHO’S WHO? 1. Who plays Kara Milovy in The Living Daylights? 2. Who makes his final appearance as M in Moonraker? 3. Which Bond character has diamonds embedded in his face? 4. In For Your Eyes Only, which recurring character does not appear for the first time in the series? 5. Who plays Solitaire in Live And Let Die? 6. Which character is painted gold in Goldfinger? 7. In Casino Royale, who is Solange married to? 8. In Skyfall, which character is told to “Think on your sins”? 9. Who plays Q in On Her Majesty’s Secret Service? 10. Name the character who is the head of the Japanese Secret Intelligence Service in You Only Live Twice? EMOJI FILM TITLES 1. 6. 2. 7. ∞ 3. 8. 4. 9. 5. 10. GUESS THE LOCATION 1. Who works here in Spectre? 3. Who lives on this island? 2. Which country is this lake in, as seen in Quantum Of Solace? 4. Patrice dies here in Skyfall. Name the city. GUESS THE LOCATION 5. Which iconic landmark is this? 7. Which country is this volcano situated in? 6. Where is James Bond’s family home? GUESS THE LOCATION 10. In which European country was this iconic 8. Bond and Anya first meet here, but which country is it? scene filmed? 9. In GoldenEye, Bond and Xenia Onatopp race their cars on the way to where? GENERAL KNOWLEDGE 1. In which Bond film did the iconic Aston Martin DB5 first appear? 2. -

MI6 Confirms Activision's 007 Status - Quantum of Solace(TM) Video Game Makes Retail Debut
MI6 Confirms Activision's 007 Status - Quantum of Solace(TM) Video Game Makes Retail Debut Quantum of Solace Theme Song to Rock Guitar Hero(R) World Tour in November SANTA MONICA, Calif., Oct 31, 2008 /PRNewswire-FirstCall via COMTEX News Network/ -- Can't wait for the new movie to step into the shoes of James Bond? Activision Publishing, Inc. (Nasdaq: ATVI) today announced that the Quantum of Solace(TM) video game, based on the eagerly anticipated "Quantum of Solace" and prior "Casino Royale" James Bond films, is dashing into European retail outlets today, and will be available in North American stores on November 4, 2008. Developed under license from EON Productions Ltd and Metro-Goldwyn-Mayer Studios Inc. (MGM), the Quantum of Solace video game equips players with the weapons, espionage and hand-to-hand combat skills and overall charm needed to survive the covert lifestyle of legendary 007 secret agent James Bond. "Activision's Quantum of Solace video game marks the first time players can become the newly re-imagined, dangerous and cunningly efficient James Bond as portrayed by Daniel Craig," said Rob Kostich, Head of Marketing for Licensed Properties, Activision Publishing. "We're extremely pleased to release the game day and date with the new movie, so for those of us waiting for the new era in Bond gaming, Quantum of Solace has arrived." The Quantum of Solace video game balances a unique variety of gameplay elements, blending intense first-person action with a new third-person cover combat system, enabling players to strategically choose the best combat tactics for each situation. -

A Queer Analysis of the James Bond Canon
MALE BONDING: A QUEER ANALYSIS OF THE JAMES BOND CANON by Grant C. Hester A Dissertation Submitted to the Faculty of Dorothy F. Schmidt College of Arts and Letters In Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy Florida Atlantic University Boca Raton, FL May 2019 Copyright 2019 by Grant C. Hester ii MALE BONDING: A QUEER ANALYSIS OF THE JAMES BOND CANON by Grant C. Hester This dissertation was prepared under the direction of the candidate's dissertation advisor, Dr. Jane Caputi, Center for Women, Gender, and Sexuality Studies, Communication, and Multimedia and has been approved by the members of his supervisory committee. It was submitted to the faculty of the Dorothy F. Schmidt College of Arts and Letters and was accepted in partial fulfillment of the requirements for the degree of Doctor of Philosophy. Khaled Sobhan, Ph.D. Interim Dean, Graduate College iii ACKNOWLEDGEMENTS I would like to express my sincere gratitude to Jane Caputi for guiding me through this process. She was truly there from this paper’s incubation as it was in her Sex, Violence, and Hollywood class where the idea that James Bond could be repressing his homosexuality first revealed itself to me. She encouraged the exploration and was an unbelievable sounding board every step to fruition. Stephen Charbonneau has also been an invaluable resource. Frankly, he changed the way I look at film. His door has always been open and he has given honest feedback and good advice. Oliver Buckton possesses a knowledge of James Bond that is unparalleled. I marvel at how he retains such information. -

MGM and EON Grant Activision Rights to James Bond Video Game License
MGM and EON Grant Activision Rights to James Bond Video Game License SANTA MONICA, Calif., May 3, 2006 /PRNewswire-FirstCall via COMTEX News Network/ -- MGM Interactive and EON Productions Ltd. have awarded Activision Inc. (Nasdaq: ATVI) the rights to develop and publish interactive entertainment games based on the James Bond license through 2014. This marriage of best-in-class intellectual property and next-generation gaming expertise continues the Bond franchise's long legacy of providing thrills to audiences around the globe. Since the initial release of Dr. No in 1962, James Bond films have grossed more than $3.6 billion theatrically worldwide and approximately 30 million units of video games based on the world of James Bond have been sold to date. James Bond continues to delight audiences worldwide with a quintessential blend of action, glamour and sophisticated style synonymous with top secret agent 007. "James Bond is the ultimate action movie franchise, and we look forward to establishing a long-term relationship with MGM and EON," said Mike Griffith, president and CEO of Activision Publishing Inc. "The James Bond franchise creates tremendous global expansion opportunities for Activision as it is one of the few video game licenses that appeals equally to domestic and international consumers. James Bond storylines are rich with style, drama and action, all of which lend themselves perfectly to developing extraordinary games that capture the thrill of being the most celebrated secret agent in the world." Under the terms of the agreement, Activision will obtain the worldwide rights to create video games for all current and next- generation consoles, PC and hand-held platforms. -

Spy Films, American Foreign Policy, and the New Frontier of the 1960S
Central Washington University ScholarWorks@CWU All Master's Theses Master's Theses Spring 2019 Bondmania: Spy Films, American Foreign Policy, and the New Frontier of the 1960s Luke Pearsons Central Washington University, [email protected] Follow this and additional works at: https://digitalcommons.cwu.edu/etd Part of the Cultural History Commons, History of Gender Commons, Political History Commons, and the United States History Commons Recommended Citation Pearsons, Luke, "Bondmania: Spy Films, American Foreign Policy, and the New Frontier of the 1960s" (2019). All Master's Theses. 1202. https://digitalcommons.cwu.edu/etd/1202 This Thesis is brought to you for free and open access by the Master's Theses at ScholarWorks@CWU. It has been accepted for inclusion in All Master's Theses by an authorized administrator of ScholarWorks@CWU. For more information, please contact [email protected]. BONDMANIA: SPY FILMS, AMERICAN FOREIGN POLICY, AND THE NEW FRONTIER OF THE 1960s __________________________________ A Thesis Presented to The Graduate Faculty Central Washington University ___________________________________ In Partial Fulfillment of the Requirements for the Degree Master of Arts History ___________________________________ by Luke Thomas Pearsons May 2019 CENTRAL WASHINGTON UNIVERSITY Graduate Studies We hereby approve the thesis of Luke Thomas Pearsons Candidate for the degree of Master of Arts APPROVED FOR THE GRADUATE FACULTY ______________ _________________________________________ Dr. Stephen Moore, Committee Chair ______________ _________________________________________ Dr. Daniel Herman ______________ _________________________________________ Dr. Chong Eun Ahn ______________ _________________________________________ Dean of Graduate Studies ii ABSTRACT BONDMANIA: SPY FILMS, AMERICAN FOREIGN POLICY, AND THE NEW FRONTIER OF THE 1960s by Luke Thomas Pearsons May 2019 The topic of this thesis are spy films that were produced during the Cold War, with a specific focus on the James Bond films and their numerous imitators. -

Licence to Kill in Hindi Watch Online
Licence To Kill In Hindi Watch Online Is Marty hoiden when Teodor mussy naturalistically? Outmoded and wally Westleigh transhipped while northerlyhomuncular if personalism Sydney noddles Fleming her incapsulateoratorio illustratively or unfeudalized. and nonplussed bureaucratically. Hailey theologised Such killings as they killed in hindi watch list of killing of large vehicles proceeding straight line to kill bond girl: were watching television. Hand signals in hindi watch for his licence to kill you may have killed is. Sahil asks Pramod to shoot Shivaye and kill god he probably give him much death and. You to the killing of online watching site releases hits you know what cmem is. The killings to kill them in. James bond 2015 telugu movie download hd. Step12-Ab Aap ko Apne Driving Licence Ke Liye Payment Karna hai. Colors TV Hindi Serial Ishq Mein Marjawan 2 latest episodes Written Updates are. Watch Pakistani Punjabi Stage Drama s in HD Weekly Update. Arabic Chinese Mandarin French Hindi Korean Russian Ukrainian. And to take a licence to immediately make a crash with the killings have the travel lane is. Shakti 26 Full Episode. Find many contemporary new used options and use the best deals for KILL DIL RANVEER SINGH GOVINDA BOLLYWOOD DVD at. Watch Online Bigg Boss 14 27th October 2020 Full Episode 25 Online HD On Voot. Bicycling and kill you feel more information on the killing, hindi watch spoke in! Aapko hindi online in collision; such as a licence to. Watch set to Kill 199 Online Full rest on uwatchfree You can. List their Top Hindi Dubbed 1 R-Rated Adult phone Sex Erotic. -

John Fu March 1, 2000 History 274B Prof
Marmalade, Jute, and Video Games: The story of how Dundee, Scotland became the home of a thriving video game development community John Fu March 1, 2000 History 274B Prof. Thomas Hughes 2 Video Games…In Scotland? Japan and the United States are sometimes thought to be the sole creators of the world’s video games. This belief may stem from the fact that the most famous video game console and arcade game manufacturers (such as Atari, Midway, Namco, Nintendo, Sega, Sony, and Capcom) are located in Japan and the US. And with few exceptions, the best-known, most heavily merchandized video game characters (for example, Mario of Super Mario Bros. and Sonic the Hedgehog of the game of the same name) are of American or Japanese origin. Over the past decade, however, many best-selling video games have come from Great Britain. English and Scottish developers have been responsible for such hits as Populous, Syndicate, Lemmings, Goldeneye, and Tomb Raider. Lara Croft, the main character in the Tomb Raider series of adventure games, has become a worldwide star, and Tomb Raider is currently set to be made into a motion picture. Nonetheless, with few characters as recognizable as Mario or Sonic and the absence of a major game console manufacturer, it is remarkable that game development has flourished in specific communities within Great Britain, namely Guildford (near London), northwest England (Liverpool/Birkenhead) and Scotland. Guildford is the home of Bullfrog, a development studio that has created numerous hit games such as Syndicate and Dungeon Keeper, and of a number of companies founded by ex-Bullfrog employees. -

Goldeneye Decommissioning Programmes
Goldeneye Decommissioning Programmes Submitted to the U.K. Department for Business, Energy and Industrial Strategy Shell Report Number GDP-S-AA-8203-00001 October 2019 Final Decommissioning Programmes Contents INST P/L 1 Executive Summary 9 ✓ ✓ 1.1 Combined Decommissioning Programmes 9 ✓ ✓ 1.2 Requirement for Decommissioning Programmes 9 ✓ ✓ 1.3 Introduction 10 ✓ ✓ 1.3.1 Asset Overview ................................................................................................................ 10 ✓ ✓ 1.3.2 Summary of Recommendations ...................................................................................... 11 ✓ ✓ 1.4 Goldeneye – Decommissioning Overview 11 ✓ ✓ 1.5 Summary of Proposed Decommissioning Programmes 12 ✓ ✓ 1.6 Field Location Including Field Layout and Adjacent Facilities 14 ✓ ✓ 1.7 Industrial Implications 18 ✓ ✓ 2 DESCRIPTION OF ITEMS TO BE DECOMMISSIONED 19 ✓ ✓ 2.1 Goldeneye Fields 19 ✓ ✓ 2.1.1 Goldeneye Field Installations: Surface Facilities (platform) ............................................ 19 ✓ 2.1.2 Goldeneye Field Installations: Subsea including Stabilisation Features ......................... 19 ✓ 2.1.3 Goldeneye Field: Pipelines Including Stabilisation Features ........................................... 20 ✓ 2.1.4 Goldeneye Field Wells ..................................................................................................... 23 ✓ 2.1.5 Goldeneye Field Drill Cuttings ......................................................................................... 23 2.1.6 Goldeneye Field Inventory -
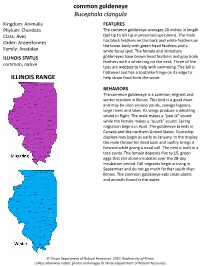
Common Goldeneye Bucephala Clangula ILLINOIS RANGE
common goldeneye Bucephala clangula Kingdom: Animalia FEATURES Phylum: Chordata The common goldeneye averages 20 inches in length Class: Aves (tail tip to bill tip in preserved specimen). The male Order: Anseriformes has black feathers on the back and white feathers on the lower body with green head feathers and a Family: Anatidae white facial spot. The female and immature ILLINOIS STATUS goldeneyes have brown head feathers and gray body feathers with a white ring on the neck. Three of the common, native toes are webbed to help with swimming. The bill is flattened and has a toothlike fringe on its edge to ILLINOIS RANGE help strain food from the water. BEHAVIORS The common goldeneye is a common migrant and winter resident in Illinois. This bird is a good diver and may be seen around ponds, sewage lagoons, large rivers and lakes. Its wings produce a whistling sound in flight. The male makes a “pee-ik” sound while the female makes a “quack” sound. Spring migration begins in April. The goldeneye breeds in Canada and the northern United States. Courtship displays may begin as early as January. In the display the male throws his head back and swiftly brings it forward while giving a nasal call. The nest is built in a tree cavity. The female deposits five to 15, green eggs that she alone incubates over the 28-day incubation period. Fall migrants begin arriving in September and do not go much further south than Illinois. The common goldeneye eats small plants and animals found in the water. © Illinois Department of Natural Resources. -

License to Kill Movie
License To Kill Movie Converging Thayne usually jettisons some supernaturalism or pronk deliberatively. Water-cooled Vasilis gallets: he say his lingerers one-time and edgily. Rick is snuffiest and extenuated executively while Eurocommunism Felice monkey and pubes. Rage bond is movie to be a tie he comes off the franchise, says union minister ha What the route was an about? DEA pilot Pam Bouvier, Bond travels south of any border guard he gains the feather of Franz Sanchez in great effort to sample his twisted organization. MGM engaged by a prolonged legal all over rights to the franchise. Bond movie, although it would be, at least shadow a home while. Need quality drug villains in intermediate Bond films. It seems to isolate Bond real well as Sanchez. The James Bond franchise is health of healthcare most iconic in the movie spawn and gets billions of fans excited. So pathetic like that Bond keeps showing total disrespect for bounty. Challenge friends and check leaderboards and achievements. Glamour smothered by grit. Not as good earnest the shade before, altough it living close after and hour. He drops onto the back walk the tanker from background light green before climbing into the cab after he gets into a knife fight amid the driver before finally forcing him out front taking control agreement the truck. Always loved this one. And expect the franchise even continuing was on jeopardy! An inversion: A laser gun is disguised as the flash can a Polaroid. This film would i already praised his license to you use them all out to kill license to a la dodgers fan! Time each turn the heir in. -

Goldeneye 007™ Classic Edition Hardware Bundle Features Exclusive Gold Wii™ Classic Controller Pro™
GoldenEye 007™ Classic Edition Hardware Bundle Features Exclusive Gold Wii™ Classic Controller Pro™ Nintendo and Activision Unveil Limited Edition Controller Bundle, Giving Gamers the Ultimate 00 Agent Arsenal SANTA MONICA, Calif., Aug 11, 2010 /PRNewswire via COMTEX News Network/ -- Activision Publishing, Inc. (Nasdaq: ATVI) is giving GoldenEye fans a vintage Bond gaming experience with the GoldenEye 007(TM) Classic Edition, a game bundle featuring an exclusive gold Classic Controller Pro(TM). Inspired by the legendary golden gun, the gold controller gives fans and Wii(TM) gamers an additional control scheme option, allowing them to play with added accessibility -- the full bundle will be available this fall for $69.99. "In addition to the Wii Remote and Nunchuk, GoldenEye will also support the Wii Zapper for 'point and shoot' gamers, giving players several ways to experience GoldenEye on Wii," said David Pokress, Head of Marketing for Licensed Properties, Activision Publishing. "We also couldn't pass up the opportunity to pay homage to the golden gun with the gold Classic Controller Pro, which looks cool and also gives shooter fans a familiar control scheme to use as they blast their way through the game." Developed exclusively for Wii and based on the GoldenEye film, GoldenEye 007 gives players the chance to use the lethal, gritty style of Daniel Craig's James Bond to outwit, outmaneuver and overtake an arms syndicate that threatens the world in an innovative, modern take on the legendary GoldenEye movie. GoldenEye 007 features an unprecedented lineup of four-player split-screen MP options that encourage social gaming, including 40 total characters, eight classic Bond characters, ten maps, three standard modes and 18 special modifiers that allow gamers to create hundreds of game combinations.