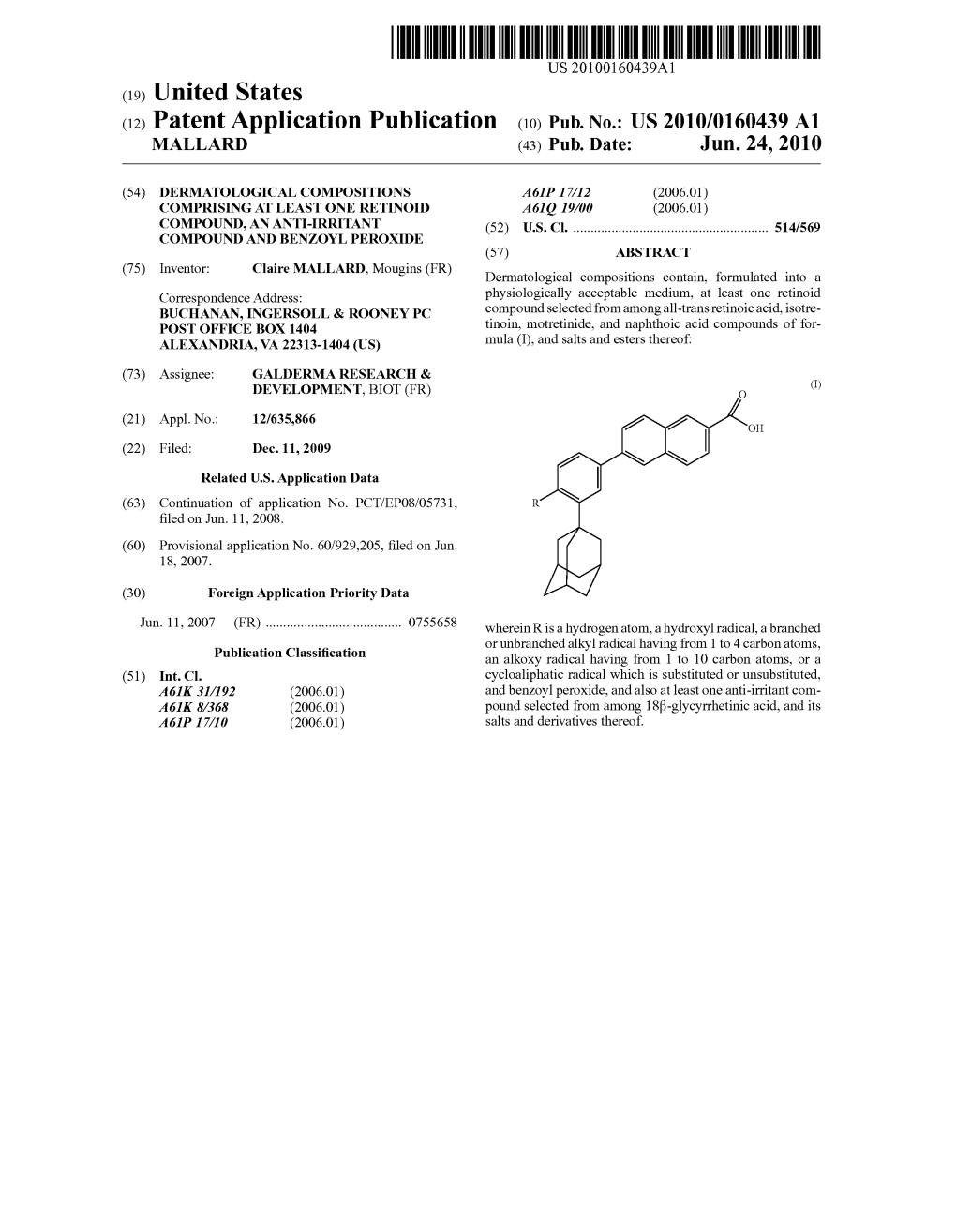
(12) Patent Application Publication (10) Pub. No.: US 2010/0160439 A1 MALLARD (43) Pub
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Centre for Reviews and Dissemination
Management of acne Lehmann H P, Andrews J S, Robinson K A, Holloway V L, Goodman S N Authors' objectives To provide a comprehensive review on the management of acne. Searching The search utilised several electronic databases from inception to 1999, including the CENTRAL Register in the Cochrane Library, MEDLINE, OLDMEDLINE (from 1960 to 1965), EMBASE, CINAHL and PsycINFO amongst others. Details of the searches were given in the report. In addition, the reference lists from key articles were checked and key experts were consulted. Only full articles published in English were eligible for the review. Study selection Study designs of evaluations included in the review Only randomised controlled trials (RCTs) or quasi-RCTs were considered for the review. There were 259 (94%) studies of a parallel-group design, 15 (5%) crossover, 30 (11%) 'split-face', and 22 (8%) parallel studies with matched controls. Specific interventions included in the review Studies of first-, second- and third-line treatments for acne were eligible for inclusion in the review. The included studies were of 140 different treatments, which were classified as: cleansers, keratolytics, topical antibacterials, keratolytic/topical antibacterial combinations, topical retinoids, topical antibacterial/retinoid combinations, oral antibacterials, oral antibacterial/keratolytics, oral antibacterial/topical retinoids, oral retinoids, anti-androgens and other. All individual treatments are listed in the review. No single control intervention was specified as an inclusion criterion; 250 different pairwise comparisons were included in the review. Participants included in the review Patients with acne were included in the review. Studies of patients with complicating co-morbidities such as endocrinopathies, and specifically, chloracne, rosacea, acne venanta, acne fulminans and acne necroticans, were excluded. -

NG198 Evidence Review F2
FINAL Management options for moderate to severe acne – pairwise comparisons GRADE tables for review question: What is the effectiveness and acceptability of interventions for the treatment of moderate to severe acne (side effects and participant reported improvement)? Oral antibiotics Table 5: Clinical evidence profile for comparison of oral antibiotic versus placebo in participants with moderate to severe acne Quality assessment No of patients Effect Importanc Quality e No of Risk of Other Oral Placeb Relative Design Inconsistency Indirectness Imprecision Absolute studies bias considerations antibiotic o (95% CI) Skin irritation - Minocycline versus placebo 11 randomised serious7 no serious no serious very serious8 none 5/119 1/55 RR 2.31 (0.28 to 24 more per 1000 (from 13 ⊕ΟΟΟ CRITICAL trials inconsistency indirectness (4.2%) (1.8%) 19.31) fewer to 333 more) VERY LOW GI side effects 51,2,3,4,5 randomised very serious10 no serious very serious8 none 82/1543 41/1177 RR 1.11 (0.62 to 4 more per 1000 (from 13 ⊕ΟΟΟ CRITICAL trials serious9 indirectness (5.3%) (3.5%) 2) fewer to 35 more) VERY LOW Thrush / candiasis - Sarecyline versus placebo 24,5 randomised serious7 no serious no serious serious11 none 4/994 0/996 Peto OR 7.44 - ⊕⊕ΟΟ CRITICAL trials inconsistency indirectness (0.4%) (0%) (1.05 to 52.86) LOW Patient reported improvement - Tetracycline versus placebo 16 randomised very no serious no serious no serious none 21/29 1/29 RR 21 (3.02 to 690 more per 1000 (from ⊕⊕ΟΟ CRITICAL trials serious9 inconsistency indirectness imprecision (72.4%) (3.4%) 145.98) 70 more to 1000 more) LOW CI: confidence interval; GI: gastrointestinal; POR: peto odds ratio; RR: risk ratio 1 Stewart 2006 Acne vulgaris: evidence reviews for management options for moderate to severe acne – pairwise comparisons FINAL (June 2021) 143 FINAL Management options for moderate to severe acne – pairwise comparisons 2 Dubertret 2003 3 Leyden 2018 4 Moore 2018 (SC1401) 5 Moore 2018 (SC1402) 6 Braathen 1984 7 Overall risk of bias judgement: serious risk of bias. -

A Comprehensive Review of Acne Vulgaris AK Mohiuddin1* 1Department of Pharmacy, World University of Bangladesh
Symbiosis ISSN Online: 2378-1726 www.symbiosisonlinepublishing.com Review Article Clinical Research in Dermatology: Open Access Open Access A Comprehensive Review of Acne Vulgaris AK Mohiuddin1* 1Department of Pharmacy, World University of Bangladesh Received: May 25, 2019; Accepted: June 6, 2019; Published: June 17, 2019 *Corresponding author: AK Mohiuddin, Assistant Professor, Department of Pharmacy, World University of Bangladesh, 151/8, Green Road, Dhanmondi, Dhaka – 1205, Bangladesh. E-mail: [email protected]; Orcid Id: https://orcid.org/0000-0003-1596-9757. Abstract Acne, also known as acne vulgaris (AV), is a long-term skin disease that occurs when hair follicles are clogged with dead skin cells and oil from the skin. It is characterized by blackheads or whiteheads, pimples, oily skin, and possible scarring. An intact stratum corneum and barrier, normal natural moisturizing factor and hyaluronic acid levels, normal Aquaporin-3 (AQP3) expression (localized at the basal lateral membranes of collecting duct cells in the kidney), and balanced sebum secretion are qualities of the skin that fall in the middle of the oily–dry spectrum. Patients rarely, if ever, complain about reduced sebum production, but elevated sebum production, yielding oily skin that can be a precursor to acne, is a common Propionibacterium acnes in adolescence, under the complaint. Several factors are known to influence sebum production. AV is mostly triggered by impact,influence as of sebum normal levels circulating are usually dehydroepiandrosterone low in childhood, rise (DHEA). in the middle-to-late It is a very common teen years, skin disorder and remain which stable can presentinto the withseventh inflammatory and eighth and decades non- untilinflammatory endogenous lesions androgen chiefly synthesis on the face dwindles. -

Pairwise Comparisons
National Institute for Health and Care Excellence Final Acne vulgaris: management [E2] Management options for mild to moderate acne – pairwise comparisons NG198 Evidence review underpinning recommendations 1.5.1, 1.5.2 and 1.5.5 to 1.5.14 (excluding 1.5.6 which is underpinned by evidence review L, 1.5.10 and bullet points 2 and 3 of recommendation 1.5.12 which are underpinned by evidence review F1 and 3 research recommendations in the NICE guideline - see evidence review E1 for the committee’s discussion of the evidence) June 2021 Final These evidence reviews were developed by the National Guideline Alliance which is a part of the Royal College of Obstetricians and Gynaecologists FINAL Error! No text of specified style in document. Disclaimer The recommendations in this guideline represent the view of NICE, arrived at after careful consideration of the evidence available. When exercising their judgement, professionals are expected to take this guideline fully into account, alongside the individual needs, preferences and values of their patients or service users. The recommendations in this guideline are not mandatory and the guideline does not override the responsibility of healthcare professionals to make decisions appropriate to the circumstances of the individual patient, in consultation with the patient and/or their carer or guardian. Local commissioners and/or providers have a responsibility to enable the guideline to be applied when individual health professionals and their patients or service users wish to use it. They should do so in the context of local and national priorities for funding and developing services, and in light of their duties to have due regard to the need to eliminate unlawful discrimination, to advance equality of opportunity and to reduce health inequalities. -

Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DIX to the HTSUS—Continued
20558 Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DEPARMENT OF THE TREASURY Services, U.S. Customs Service, 1301 TABLE 1.ÐPHARMACEUTICAL APPEN- Constitution Avenue NW, Washington, DIX TO THE HTSUSÐContinued Customs Service D.C. 20229 at (202) 927±1060. CAS No. Pharmaceutical [T.D. 95±33] Dated: April 14, 1995. 52±78±8 ..................... NORETHANDROLONE. A. W. Tennant, 52±86±8 ..................... HALOPERIDOL. Pharmaceutical Tables 1 and 3 of the Director, Office of Laboratories and Scientific 52±88±0 ..................... ATROPINE METHONITRATE. HTSUS 52±90±4 ..................... CYSTEINE. Services. 53±03±2 ..................... PREDNISONE. 53±06±5 ..................... CORTISONE. AGENCY: Customs Service, Department TABLE 1.ÐPHARMACEUTICAL 53±10±1 ..................... HYDROXYDIONE SODIUM SUCCI- of the Treasury. NATE. APPENDIX TO THE HTSUS 53±16±7 ..................... ESTRONE. ACTION: Listing of the products found in 53±18±9 ..................... BIETASERPINE. Table 1 and Table 3 of the CAS No. Pharmaceutical 53±19±0 ..................... MITOTANE. 53±31±6 ..................... MEDIBAZINE. Pharmaceutical Appendix to the N/A ............................. ACTAGARDIN. 53±33±8 ..................... PARAMETHASONE. Harmonized Tariff Schedule of the N/A ............................. ARDACIN. 53±34±9 ..................... FLUPREDNISOLONE. N/A ............................. BICIROMAB. 53±39±4 ..................... OXANDROLONE. United States of America in Chemical N/A ............................. CELUCLORAL. 53±43±0 -

Acne Protection: Measures and Miseries
Vol 8, Issue 1, 2020 ISSN - 2347-5536 Review Article ACNE PROTECTION: MEASURES AND MISERIES ABDUL KADER MOHIUDDIN* Secretary and Treasurer, Dr. M. Nasirullah Memorial Trust, Tejgaon, Dhaka 1215, Bangladesh. Email: [email protected] Received: 14 October 2019, Revised and Accepted: 23 December 2019 ABSTRACT Acne, also known as acne vulgaris (AV), is a long-term skin disease that occurs when hair follicles are clogged with dead skin cells and oil from the skin. It is characterized by blackheads or whiteheads, pimples, oily skin, and possible scarring. An intact stratum corneum and barrier, normal natural moisturizing factor and hyaluronic acid levels, normal Aquaporin-3 expression (localized at the basal lateral membranes of collecting duct cells in the kidney), and balanced sebum secretion are qualities of the skin that fall in the middle of the oily-dry spectrum. Patients rarely, if ever, complain about reduced sebum production, but elevated sebum production, yielding oily skin that can be a precursor to acne, is a common complaint. Several factors are known to influence sebum production. AV is mostly triggered by Propionibacterium acnes in adolescence, under the influence of normal circulating dehydroepiandrosterone (DHEA). It is a very common skin disorder which can present with inflammatory and non-inflammatory lesions chiefly on the face but can also occur on the upper arms, trunk, and back. Age, in particular, has a significant and well-known impact, as sebum levels are usually low in childhood, rise in the middle to late teen years, and remain stable into the seventh and eighth decades until endogenous androgen synthesis dwindles. -

NG198 Evidence Review F1
FINAL Management options for people with moderate to severe acne vulgaris - network meta-analyses Clinical studies The excluded studies list below relates to all evidence reviews that used the same search output and these are studies that are excluded from all of the following reviews: mild-to- moderate NMA, moderate-to-severe NMA, mild-to-moderate pairwise and moderate-to- severe pairwise reports, as well as from refractory acne, maintenance of acne and polycystic ovary syndrome reports. Table 24: Excluded clinical studies and reasons for their exclusion Reference Reason for exclusion Abbasi, M. A. K., A., Aziz ur, Rehman, Saleem, H.,Jahangir, S. No relevant study M.,Siddiqui, S. Z.,Ahmad, V. U.Preparation of new formulations of population - sample anti-acne creams and their efficacy. 2010. African Journal of includes people with mild Pharmacy and Pharmacology to severe acne and study is not relevant for PCOS, maintenance or refractory treatments Abdel Hay, R. H., R.,Abdel Hady, M.,Saleh, N.Clinical and Reported outcomes dermoscopic evaluation of combined (salicylic acid 20% and azelaic relevant for the network acid 20%) versus trichloroacetic acid 25% chemical peel in acne: an meta-analysis but not in RCT. 2019. Journal of Dermatological Treatment enough detail to include in the analysis. Outcomes were not relevant for pairwise comparisons - including PCOS, maintenance and refractory treatments Abdel Meguid, A. M. A. E. A. A., D.,Omar, H.Trichloroacetic acid Reported outcomes versus salicylic acid in the treatment of acne vulgaris in dark-skinned relevant for the network patients. 2015. Dermatologic Surgery meta-analysis but not in enough detail to include in the analysis. -

(12) Patent Application Publication (10) Pub. No.: US 2003/0068365A1 Suvanprakorn Et Al
US 2003.0068365A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2003/0068365A1 Suvanprakorn et al. (43) Pub. Date: Apr. 10, 2003 (54) COMPOSITIONS AND METHODS FOR Related U.S. Application Data ADMINISTRATION OF ACTIVE AGENTS USING LIPOSOME BEADS (60) Provisional application No. 60/327,643, filed on Oct. 5, 2001. (76) Inventors: Pichit Suvanprakorn, Bangkok (TH); Tanusin Ploysangam, Bangkok (TH); Publication Classification Lerson Tanasugarn, Bangkok (TH); Suwalee Chandrkrachang, Bangkok (51) Int. Cl." .......................... A61K 9/127; A61K 35/78 (TH); Nardo Zaias, Miami Beach, FL (52) U.S. Cl. ............................................ 424/450; 424/725 (US) (57) ABSTRACT Correspondence Address: Law Office of Eric G. Masamori Compositions and methods for administration of active 6520 Ridgewood Drive agents encapsulated within liposome beads to enable a wider Castro Valley, CA 94.552 (US) range of delivery vehicles, to provide longer product shelf life, to allow multiple active agents within the composition, (21) Appl. No.: 10/264,205 to allow the controlled use of the active agents, to provide protected and designable release features and to provide (22) Filed: Oct. 3, 2002 Visual inspection for damage and inconsistency. US 2003/0068365A1 Apr. 10, 2003 COMPOSITIONS AND METHODS FOR toxic degradation of the products, leakage of the drug from ADMINISTRATION OF ACTIVE AGENTS USING the liposome and the modifications of the Size and morphol LPOSOME BEADS ogy of the phospholipid liposome vesicles through aggre gation and fusion. Liposome vesicles are known to be CROSS REFERENCE TO OTHER thermodynamically relatively unstable at room temperature APPLICATIONS and can Spontaneously fuse into larger, leSS Stable altered liposome forms. -

Stembook 2018.Pdf
The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances FORMER DOCUMENT NUMBER: WHO/PHARM S/NOM 15 WHO/EMP/RHT/TSN/2018.1 © World Health Organization 2018 Some rights reserved. This work is available under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 IGO licence (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/igo). Under the terms of this licence, you may copy, redistribute and adapt the work for non-commercial purposes, provided the work is appropriately cited, as indicated below. In any use of this work, there should be no suggestion that WHO endorses any specific organization, products or services. The use of the WHO logo is not permitted. If you adapt the work, then you must license your work under the same or equivalent Creative Commons licence. If you create a translation of this work, you should add the following disclaimer along with the suggested citation: “This translation was not created by the World Health Organization (WHO). WHO is not responsible for the content or accuracy of this translation. The original English edition shall be the binding and authentic edition”. Any mediation relating to disputes arising under the licence shall be conducted in accordance with the mediation rules of the World Intellectual Property Organization. Suggested citation. The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances. Geneva: World Health Organization; 2018 (WHO/EMP/RHT/TSN/2018.1). Licence: CC BY-NC-SA 3.0 IGO. Cataloguing-in-Publication (CIP) data. -

A Abacavir Abacavirum Abakaviiri Abagovomab Abagovomabum
A abacavir abacavirum abakaviiri abagovomab abagovomabum abagovomabi abamectin abamectinum abamektiini abametapir abametapirum abametapiiri abanoquil abanoquilum abanokiili abaperidone abaperidonum abaperidoni abarelix abarelixum abareliksi abatacept abataceptum abatasepti abciximab abciximabum absiksimabi abecarnil abecarnilum abekarniili abediterol abediterolum abediteroli abetimus abetimusum abetimuusi abexinostat abexinostatum abeksinostaatti abicipar pegol abiciparum pegolum abisipaaripegoli abiraterone abirateronum abirateroni abitesartan abitesartanum abitesartaani ablukast ablukastum ablukasti abrilumab abrilumabum abrilumabi abrineurin abrineurinum abrineuriini abunidazol abunidazolum abunidatsoli acadesine acadesinum akadesiini acamprosate acamprosatum akamprosaatti acarbose acarbosum akarboosi acebrochol acebrocholum asebrokoli aceburic acid acidum aceburicum asebuurihappo acebutolol acebutololum asebutololi acecainide acecainidum asekainidi acecarbromal acecarbromalum asekarbromaali aceclidine aceclidinum aseklidiini aceclofenac aceclofenacum aseklofenaakki acedapsone acedapsonum asedapsoni acediasulfone sodium acediasulfonum natricum asediasulfoninatrium acefluranol acefluranolum asefluranoli acefurtiamine acefurtiaminum asefurtiamiini acefylline clofibrol acefyllinum clofibrolum asefylliiniklofibroli acefylline piperazine acefyllinum piperazinum asefylliinipiperatsiini aceglatone aceglatonum aseglatoni aceglutamide aceglutamidum aseglutamidi acemannan acemannanum asemannaani acemetacin acemetacinum asemetasiini aceneuramic -

NG198 Evidence Review F2
FINAL Management options for moderate to severe acne – pairwise comparisons Clinical studies The excluded studies list below relates to all evidence reviews that used the same search output and these are studies that are excluded from all of the following reviews: mild-to- moderate NMA, moderate-to-severe NMA, mild-to-moderate pairwise and moderate-to- severe pairwise reports, as well as from refractory acne, maintenance of acne and polycystic ovary syndrome reports. Table 14: Excluded clinical studies and reasons for their exclusion Reference Reason for exclusion Abbasi, M. A. K., A., Aziz ur, Rehman, Saleem, H.,Jahangir, S. No relevant study M.,Siddiqui, S. Z.,Ahmad, V. U.Preparation of new formulations of population - sample anti-acne creams and their efficacy. 2010. African Journal of includes people with mild Pharmacy and Pharmacology to severe acne and study is not relevant for PCOS, maintenance or refractory treatments Abdel Hay, R. H., R.,Abdel Hady, M.,Saleh, N.Clinical and Reported outcomes dermoscopic evaluation of combined (salicylic acid 20% and azelaic relevant for the network acid 20%) versus trichloroacetic acid 25% chemical peel in acne: an meta-analysis but not in RCT. 2019. Journal of Dermatological Treatment enough detail to include in the analysis. Outcomes were not relevant for pairwise comparisons - including PCOS, maintenance and refractory treatments Abdel Meguid, A. M. A. E. A. A., D.,Omar, H.Trichloroacetic acid Reported outcomes versus salicylic acid in the treatment of acne vulgaris in dark-skinned relevant for the network patients. 2015. Dermatologic Surgery meta-analysis but not in enough detail to include in the analysis. -

Other Review(S)
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 211527Orig1s000 OTHER REVIEW(S) MEMORANDUM REVIEW OF REVISED LABEL AND LABELING Division of Medication Error Prevention and Analysis (DMEPA) Office of Medication Error Prevention and Risk Management (OMEPRM) Office of Surveillance and Epidemiology (OSE) Center for Drug Evaluation and Research (CDER) Date of This Memorandum: October 1, 2019 Requesting Office or Division: Division of Dermatology and Dental Products (DDDP) Application Type and Number: NDA 211527 Product Name and Strength: Aklief (trifarotene) cream, 0.005% Applicant/Sponsor Name: Galderma Research and Development, LLC FDA Received Date: August 30, 2019 and September 13, 2019 OSE RCM #: 2018-2153-2 DMEPA Safety Evaluator: Madhuri R. Patel, PharmD DMEPA Team Leader: Sevan Kolejian, PharmD, MBA 1 PURPOSE OF MEMORANDUM The Applicant submitted revised 30 gram container label, Prescribing Information (PI) and Patient Package Insert (PPI) received on August 30, 2019 and revised container labels and carton labeling on September 13, 2019 for Aklief. Division of Dermatology and Dental Products (DDDP) requested that we review the revised container labels, carton labeling, Prescribing Information (PI), and Patient Package Insert (PPI) for Aklief (Appendix A) to determine if they are acceptable from a medication error perspective. The revisions are in response to recommendations that we made during a previous label and labeling review.a 2 CONCLUSION The Applicant implemented all of our recommendations and we have no additional recommendations at this time. a Patel M. Label and Labeling MEMORANDUM for Aklief (NDA 211527). Silver Spring (MD): FDA, CDER, OSE, DMEPA (US); 2019 AUG 19. RCM No.: 2018-2153-1.