Anne Constant Cohen
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pan American World Health Health Organization
executive committee of u,orking party of PAN AMERICAN WORLD ORGANIZATION ORGANIZATION _x_the HEALTHdtrecttng council the regionalHEALTHcommitte__i_,_,\fl_ 8and Meeting Washington, D.C. June-July 1979 Provisional A_enda Item 23 CE82/17 (Eng.) 17 May 1979 ORIGINAL: ENGLISH SITUATION REPORT ON PAHO'S DISASTER PREPAREDNESS PROGRAM IN THE AMERICAS Introduction The Americas are particularly vulnerable to natural disasters. Major earthquakes have occurred in Peru (1970), Nicaragua (1972), and Guatemala (1976). Volcanic activities caused evacuation of large numbers of people in Guadeloupe (1975-1976) and St. Vincent (1979). Hurricanes strike in the Caribbean and along the Atlantic Coast of Central America periodically (for example, hurricane Fifi, Honduras, 1974). Floods, on many occasions, have severely affected the economic and social develop- ment and the provision of primary health care of many countries in the Region (Paraguay, Bolivia, Dominican Republic, Brazil, Jamaica, etc.). Objectives of the Program Several resolutions on relief assistance have been adopted by the Governing Bodies of WHO and PAHO. 1 In the last three years, increasing emphasis has been placed by the Directing Council of the Pan American Health Organization on technical cooperation in emergency preparedness before disasters actually strike. 2 In October 1976 the Directing Council of PAHO, ,anxious that the internatior_l assistance given to countries affected by natural disasters should be better coordinated, rational, and more effective," requested that the Director -

Significant Data on Major Disasters Worldwide, 1900-Present
DISASTER HISTORY Signi ficant Data on Major Disasters Worldwide, 1900 - Present Prepared for the Office of U.S. Foreign Disaster Assistance Agency for International Developnent Washington, D.C. 20523 Labat-Anderson Incorporated Arlington, Virginia 22201 Under Contract AID/PDC-0000-C-00-8153 INTRODUCTION The OFDA Disaster History provides information on major disasters uhich have occurred around the world since 1900. Informtion is mare complete on events since 1964 - the year the Office of Fore8jn Disaster Assistance was created - and includes details on all disasters to nhich the Office responded with assistance. No records are kept on disasters uhich occurred within the United States and its territories.* All OFDA 'declared' disasters are included - i.e., all those in uhich the Chief of the U.S. Diplmtic Mission in an affected country determined that a disaster exfsted uhich warranted U.S. govermnt response. OFDA is charged with responsibility for coordinating all USG foreign disaster relief. Significant anon-declared' disasters are also included in the History based on the following criteria: o Earthquake and volcano disasters are included if tbe mmber of people killed is at least six, or the total nmber uilled and injured is 25 or more, or at least 1,000 people art affect&, or damage is $1 million or more. o mather disasters except draught (flood, storm, cyclone, typhoon, landslide, heat wave, cold wave, etc.) are included if the drof people killed and injured totals at least 50, or 1,000 or mre are homeless or affected, or damage Is at least S1 mi 1l ion. o Drought disasters are included if the nunber affected is substantial. -

Disaster Assistance System Historical Analysis
ORA_SIIQ _DISNO DISNO_MOD DmNOWN_NO_l BLAN!Cl _DATIIl___DmN_()~O_l DISASTIIR_NAMII LATITODII LONGITODE DmNOWN_NO_33 DISASTER_L_()_c:ATION DISNO_MAIN DmNOWN_N0_6 DmNOWN_N0_7 DmNOWN_NO_S DmNOWN_NO H ___DmNOWN_N0_35 tJNICIIOWN_N0_36 1 O*FED 2200 012578 515 NATIONWIDE ELECTRICA O*UOS 1200 012578 MEXICO FLASHFLOOD I O*UlS 1200 012378 4 O*U24 -'iioo 121277 O*USB 1203 050178 JAPAN TSUNAMI HONSHU ISLAND 60004 oc 1100 112977 7 000001 2200 050278 386 INDIA DROUGHT BENGAL 001001 2100 012578 617 UGANDA EPIDEMIC NATIONWIDE 002001 1500 092877 520 GUATEMALA VOLCANO 01475N 09154W _-~~~_MARIA __ 10 002002 1500 012578 597 ST. VINCENT VOLCANO 01482N 06117W ST PIERRE,MT PELEE 11 002003 1500 012578 547 MT SOUFRIERE ERUPT VINCENT IS 12 002006 1703 092877 388 E. PAKISTAN CYCLONE W SUNDARBANS 13 003001 1400 --~---------050178 703 CANADA LANDSLIDE __ FRANK, ALBERTA __ 14 005001 1100 012578 386 INDIA EARTHQUAKE KANGRA 15 005002 1703 3ss iE PAKISTAN CYCLON-E--~1 _______,_c:!liTTAGONG _ -~12s7s --+-- -~~-' 'E PAKISTAN CYCiONE~--- --~ 16 005003 1703 092877 388 ~~-- ---~-- CHITIAGONG --------~-17 006001 1100 050178 513 _CHILE EARTHQUAKE VALPARAIOS 18 006002 1100 111275 484 _FORMOSA_EARTHQUAKE FORMOSA 19 006003 1100 092877 598 , ECUADOR_-COLUMBIA _E_'<__~ 200 MILES OFF COAST 20 006004 1306 1 :FRANCE MINE EXPLOSN COURRIERES -__ ~ ~3-~!!771138 21 007001 2109 092877 386 INDIA B. PLAGUE __ l 22,007002 - 1702 092877 ·-----~-478 HONG KONG TYPHOON HONG KONG 23 008001 1100 --------------012578 145 . I TAL Y__ EARTHQUAKE MESSINA 24 008002 1306 121277 109 GER MINE DISASTER RADBO,~Dc_____ . - ~~--- 25,009_Q_O_l__ 1600 092877 523 MEXICO FIRE ACAPULCO 26' 009002 2109 092877 435 CHINA B.PLAGUE t---- ---- . INDIA, CHINA 27' 009003 1100 'o5047B '1ss ROMANIA EQ 28 009004 2109 092877 497 INDONESIA B. -

Is the USAF Flying Force Large Enough? Assessing Capacity Demands in Four Alternative Futures
C O R P O R A T I O N Is the USAF Flying Force Large Enough? Assessing Capacity Demands in Four Alternative Futures Alan J. Vick, Paul Dreyer, John Speed Meyers For more information on this publication, visit www.rand.org/t/RR2500 Library of Congress Cataloging-in-Publication Data is available for this publication. ISBN: 978-1-9774-0072-7 Published by the RAND Corporation, Santa Monica, Calif. © Copyright 2018 RAND Corporation R® is a registered trademark. Limited Print and Electronic Distribution Rights This document and trademark(s) contained herein are protected by law. This representation of RAND intellectual property is provided for noncommercial use only. Unauthorized posting of this publication online is prohibited. Permission is given to duplicate this document for personal use only, as long as it is unaltered and complete. Permission is required from RAND to reproduce, or reuse in another form, any of its research documents for commercial use. For information on reprint and linking permissions, please visit www.rand.org/pubs/permissions. The RAND Corporation is a research organization that develops solutions to public policy challenges to help make communities throughout the world safer and more secure, healthier and more prosperous. RAND is nonprofit, nonpartisan, and committed to the public interest. RAND’s publications do not necessarily reflect the opinions of its research clients and sponsors. Support RAND Make a tax-deductible charitable contribution at www.rand.org/giving/contribute www.rand.org Preface The 1997 National Defense Authorization Act (NDAA) directed the Department of Defense to conduct a systematic review of U.S. -

SDSR Vol04 No1.Pdf
ISSN: 1533-2535 Volume 4 No. 1 Spring 2004 Research Articles Welcome to the Spring 2004 issue of Security and Defense Avances y Límites de la Participación de México en la Studies Review, the Interdisciplinary Journal of the Center for Seguridad Hemisférica a Inicios del Siglo XXI - PDF Hemispheric Defense Studies. Raúl Benitez Manaut y Georgina Sánchez Bienvenido a la edición de primavera de 2004 de Security and Defense Studies Review, the Interdisciplinary Journal of the Abstract Biography Center for Hemispheric Defense Studies. Integración y Modelo de Seguridad Democrática en Bem vindo a edição do primavera de 2004 da Security and Centroamérica: Su Influencia dentro de la OEA – PDF Defense Studies Review, the Interdisciplinary Journal of the Mauricio Herdocia Sacasa Center for Hemispheric Defense Studies. Abstract Biography Message from the Editor A New Civil-Military Pragmatism in Latin America – PDF Editors and Editorial Board David Pion-Berlin Archive Editions Abstract Biography Fall 2003 Summer 2002 Spring 2003 Winter 2001 Winter 2002/2003 Spring 2001 Essays Security and Defense Studies Review is currently accepting Defense, Internal Security and the Other Roles of Belize’s submissions for Volume 4 No.2, Spring 2005 issue. Click Military – PDF here for more information. Dion E. Phillips Abstract Biography Security and Defense Studies Review está aceptando manuscritos para el Volumen 4 No. 2 para Primavera de 2005. Pulsa aquí para más información. By permission of Tiempos del Mundo, edition of 25 to 31 December 2003 (Year 8, Number 52), p. 15-19. “Centroamérica y el Caribe” Tiempos del mundo 25 dic 03 p 15 articles.pdf Security and Defense Studies Review esta aceitando Tiempos del mundo 25 dic 03 p 16 articles.pdf manuscritos para publicação nas edicãos para Volumen 4 No. -

Children of the Storm
Children of the Storm by Dean Koontz NO HAVEN As she and the children stood by the windows, watching the sea which glittered madly with reflected moonlight, Sonya felt more at peace than she had for a long time. The solidity of Seawatch made her feel as if she were in a fortress, sealed away from harm Alex destroyed that mood in a moment. Are you worried? he asked. Sonya did not look away from the sea. Why should I be worried? He won't hurt you. She looked at Alex. His eyes were very dark, almost too dark to see in the meager light. Who won't? He scuffed his small feet on the carpet and looked back at the rolling sea. The man. What man? You know, said Tina. The man who says he is going to kill me and Alex BOOK ONE ONE Having lived nearly all of her twenty-three years in the brief summers and the bitter winters of Maine and Massachusetts, Sonya Carter was especially intrigued by the Caribbean-by the almost too-bright skies, the warm breezes that smelled of salty ocean air, the palm trees that could be seen nearly everywhere, the delicious mangoes, the spectacular sunsets and the sudden twilights that deepened rapidly into purple darkness Too, the warmth of the Caribbean seemed to represent life, bustle, excitement, anticipation-while New England, in her mind, was associated with death and loneliness. She had lost her parents in Maine, thirteen years ago, when their car overturned on a stretch of icy highway. -
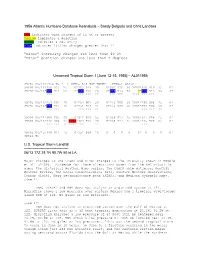
1956 Atlantic Hurricane Database Reanalysis – Sandy Delgado and Chris Landsea
1956 Atlantic Hurricane Database Reanalysis – Sandy Delgado and Chris Landsea Red indicates wind changes of 15 kt or greater Yellow indicates a deletion Green indicates a new entry Blue indicates lat/lon changes greater than 1º “Minor” intensity changes are less than 20 kt “Minor” position changes are less than 2 degrees Unnamed Tropical Storm 1 [June 12-15, 1956] – AL011956 39285 06/12/1956 M= 4 1 SNBR= 858 NOT NAMED XING=1 SSS=0 39290 06/12*220 915 25 0*225 913 30 0*231 912 30 1009*240 910 35 0* 39290 06/12*200 915 25 0*203 915 25 0*208 915 30 0*216 915 35 0* *** *** *** ** *** *** **** *** *** 39295 06/13*253 907 40 0*264 907 50 0*275 909 50 1004*290 908 45 0* 39295 06/13*230 915 40 0*251 913 45 0*275 909 50 1004*294 906 50 0* *** *** *** *** ** *** *** ** 39300 06/14*306 905 40 0*322 910 35 0*338 917 25 1006*347 928 25 0* 39300 06/14*310 906 35 1001*324 910 30 0*338 917 25 1006*345 928 25 0* *** *** ** **** *** ** *** 39305 06/15*349 933 25 0*352 938 20 0* 0 0 0 0* 0 0 0 0* 39310 TS U.S. Tropical Storm Landfall ------------------------------------- 06/13 17Z 29.1N 90.7W 50 kt LA Major changes to the track and minor changes to the intensity shown in McAdie et al. (2009). Evidence for these alterations comes from the NHC microfilm maps, the Historical Weather Maps series, the COADS ship database, Monthly Weather Review, the Local Climatological Data, Surface Weather Observations, Connor (1956), Navy reconnaissance book (ATSR), and Mexican synoptic maps. -

N O T I C E This Document Has Been Reproduced from Microfiche. Although It Is Recognized That Certain Portions Are Illegible, It
N O T I C E THIS DOCUMENT HAS BEEN REPRODUCED FROM MICROFICHE. ALTHOUGH IT IS RECOGNIZED THAT CERTAIN PORTIONS ARE ILLEGIBLE, IT IS BEING RELEASED IN THE INTEREST OF MAKING AVAILABLE AS MUCH INFORMATION AS POSSIBLE W(,-- Teshnk* Mw wWum 73288 K An Atlas of 1977 and 1978 GEOS-S Radar Altimeter Data for Tropical Cyclone Studies r A AN AUA:i uF 1971 AsD 1 y i j i;„J—.S, .0 F GEOS —.i UAVAh ALTIIETEI DATA FU., TRnt'ICAL CtCLU,4L 3TUJ1h.9 (tiP,:;A) .211 riL All/ el k tiJ 1 CjCL d4b G.i/47 2d751 H. R. Stanley and R. L. Taylor 1 c (^r_.; 1980 , August 1980 RECEIF,^VU E 01M ^cr^98n, NAM M^ National Aeronautics and Space Administration^^^^`^ °. Wanops Flight Center Wallops Island, Virg!nia 23337 AC 804 824-341 1 ^> c\2v `i - NASA Technical Memorandum 73288 An Atlas of 1977 and 1978 GEOS-3 Radar Altimeter Data for Tropical Cyclone Studies H. R. Stanley NASA Wallops Flight Center Wallops Island, Virginia 23337 and R. L. Taylor EG&G/Washington Analytical Services Center, Inc. Wolf Research and Development Group P.O. Box 476 Pocomoke City, Maryland 21851 National Aeronautics and Space Administration WbIlops Flight Center Wallops Island, Virginia 23337 AC 804 824-3411 FOREWORD This document's primary purpose is to provide the means for locating and extracting GEOS-3 altimeter data ac4uired for the analysis of specific hurricanes, typhoons, and other tropical cyclones. This data may also be extremely useful in the analysis of the behavior of the altimeter instrument in the presence of severe meteorological disturbances as well as provide a data base which can be useful in the resolution of apparently anomalous geoid or sea surface characteristics. -

Downloaded 09/23/21 06:07 PM UTC DECEMBER1956 MONTHLYWEATHERREVIEW 437
436 MONTHLY WEATHER, REVIEW DECEMBER1956 HURRICANE SEASON OF 1956 GORDON E. DUNN,WALTER R. DAVIS, AND PAUL L. MOORE Weather Bureau Office, Mlarni, Fla. TABLE1.-Damage and deaths from all tropical storms and dis- 1. GENERAL SUMMARY tnrbames of 1956 The 1956 hurricane seasonwas comparatively mild Type of storm Area from thestandpoint of stormfrequency. Only eight - ~___- tropical storms developed compared to an average during 4 La., Miss. 0 Tampico, Mex. thepast two decades of ten;four reached hurricane 18 French West Indies. 9 Puerto Rieo. intensitycompared to a normal of five duringrecent 0 Bahamas. 27 Mexico. years. In onlytwo of thepast 15 years have tropical 15 Mostly in La., Miss., Ala.. Fla. storms been so few and, by 1925 standards of detection HurricaneGreta- ...~~..~ 0 Mostly Fla. east coast. 3-5. ~.~ 1,899,201 Puerto1 Rico and other and classification, possibly only three storms would have islands in Antilles. Tropical disturbance.. ~ ~ July 4-5-. ~ ~ ~ 503,000 0 Alabama. been designated astropical. Two storms reached the &uasi-tropicalstorm...~~ Oct. 15-16." 3,000,000 2 Florida. c___~ coastline of the United States, both in theGulf of Mexico. Total in United .. .~. -.~ ~. .. ~ ~ $30,007,605 The 1956 season was also mild from the standpoint of States. TotalAtlantic I..."-.-- ..... I$67,836,806 I il I tropical stormintensity. One of thestorms was of hurricane area. I I/ hurricane intensity for only a few hours, Flossy was of *Zero indicates no deathsreported. full hurricaneintensity nomore than 24 hours,and hurricane Greta was asmuch extratropical as tropical storms and there was never any definite center or eye. -

Status and Trends of Caribbean Coral Reefs
Status and Trends of Caribbean Coral Reefs: 1970-2012 EDITED BY JEREMY JACKSON · MARY DONOVAN · KATIE CRAMER · VIVIAN LAM STATUS AND TRENDS OF CARIBBEAN CORAL REEFS: 1970-2012 EDITED BY JEREMY JACKSON, MARY DONOVAN, KATIE CRAMER, AND VIVIAN LAM Dedication: This book is dedicated to the many people who have worked on coral reefs to understand them, to protect them, and to appreciate their beauty and meaning for humanity and the natural world. We also recognize the International Coral Reef Initiative and partners, and particularly the people of all nations throughout the wider Caribbean region who continue to strive for the existence of healthy Caribbean reefs for future generations. Note: The conclusions and recommendations of this volume are solely the opinions of the authors and contributors and do not constitute a statement of policy, decision, or position on behalf of the participating organizations. Front Cover: Dead parrotfish (Sparisoma viride) caught in gillnet in front of a completely destroyed reef (Photo by Ayana Elizabeth Johnson) Back Cover: School of the stoplight parrotfish Sparisoma viride on the south shore of Bermuda. (Photo by Philipp Rouja) Citation: Jackson JBC, Donovan MK, Cramer KL, Lam VV (editors). (2014) Status and Trends of Caribbean Coral Reefs: 1970-2012. Global Coral Reef Monitoring Network, IUCN, Gland, Switzerland. Design and layout: UNITgraphics.com / Imre Sebestyén jr., Tibor Lakatos © Global Coral Reef Monitoring Network c/o International Union for the Conservation of Nature Global Marine and Polar Program 1630 Connecticut Avenue N. W. Washington, D. C. United States of America CONTENTS FOREWORD ..................................................................... 6 INTRODUCTION ................................................................. 7 ACKNOWLEDGEMENTS, CO-SPONSORS, AND SUPPORTERS OF GCRMN ...