Evaluating the Monophyly and Biogeography of Cryptantha (Boraginaceae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Two New Genera in the Omphalodes Group (Cynoglosseae, Boraginaceae)
Nova Acta Científica Compostelana (Bioloxía),23 : 1-14 (2016) - ISSN 1130-9717 ARTÍCULO DE INVESTIGACIÓN Two new genera in the Omphalodes group (Cynoglosseae, Boraginaceae) Dous novos xéneros no grupo Omphalodes (Cynoglosseae, Boraginaceae) M. SERRANO1, R. CARBAJAL1, A. PEREIRA COUTINHO2, S. ORTIZ1 1 Department of Botany, Faculty of Pharmacy, University of Santiago de Compostela, 15782 Santiago de Compostela , Spain 2 CFE, Centre for Functional Ecology, Department of Life Sciences, University of Coimbra, 3000-456 Coimbra, Portugal *[email protected]; [email protected]; [email protected]; [email protected] *: Corresponding author (Recibido: 08/06/2015; Aceptado: 01/02/2016; Publicado on-line: 04/02/2016) Abstract Omphalodes (Boraginaceae, Cynoglosseae) molecular phylogenetic relationships are surveyed in the context of the tribe Cynoglosseae, being confirmed that genusOmphalodes is paraphyletic. Our work is focused both in the internal relationships among representatives of Omphalodes main subgroups (and including Omphalodes verna, the type species), and their relationships with other Cynoglosseae genera that have been related to the Omphalodes group. Our phylogenetic analysis of ITS and trnL-trnF molecular markers establish close relationships of the American Omphalodes with the genus Mimophytum, and also with Cynoglossum paniculatum and Myosotidium hortensia. The southwestern European annual Omphalodes species form a discrete group deserving taxonomic recognition. We describe two new genera to reduce the paraphyly in the genus Omphalodes, accommodating the European annual species in Iberodes and Cynoglossum paniculatum in Mapuchea. The pollen of the former taxon is described in detail for the first time. Keywords: Madrean-Tethyan, phylogeny, pollen, systematics, taxonomy Resumo Neste estudo analisamos as relacións filoxenéticas deOmphalodes (Boraginaceae, Cynoglosseae) no contexto da tribo Cynoglosseae, confirmándose como parafilético o xéneroOmphalodes . -

COSEWIC Assessment and Status Report on the Tiny Cryptantha Cryptantha Minima in Canada
COSEWIC Assessment and Status Report on the Tiny Cryptantha Cryptantha minima in Canada THREATENED 2012 COSEWIC status reports are working documents used in assigning the status of wildlife species suspected of being at risk. This report may be cited as follows: COSEWIC. 2012. COSEWIC assessment and status report on the Tiny Cryptantha Cryptantha minima in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. x + 37 pp. (www.registrelep-sararegistry.gc.ca/default_e.cfm). Previous report(s): COSEWIC. 2000. COSEWIC assessment and status report on the tiny cryptanthe Cryptantha minima in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. vi + 18 pp. Smith, B. 1998. COSEWIC status report on the tiny cryptanthe Cryptantha minima in Canada, in COSEWIC assessment and status report on the tiny cryptanthe Cryptantha minima in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. 1-18 pp. Production note: COSEWIC would like to acknowledge Sue Michalsky for writing the status report on the Tiny Cryptantha Cryptantha minima in Canada, prepared under contract with Environment Canada. This report was overseen and edited by Bruce Bennett and Erich Haber, Co-chairs of the COSEWIC Vascular Plants Specialist Subcommittee. For additional copies contact: COSEWIC Secretariat c/o Canadian Wildlife Service Environment Canada Ottawa, ON K1A 0H3 Tel.: 819-953-3215 Fax: 819-994-3684 E-mail: COSEWIC/[email protected] http://www.cosewic.gc.ca Également disponible en français sous le titre Ếvaluation et Rapport de situation du COSEPAC sur la Cryptanthe minuscule (Cryptantha minima) au Canada. Cover illustration/photo: Tiny Cryptantha — Source: Environment Canada 2010. -

Terrestrial and Marine Biological Resource Information
APPENDIX C Terrestrial and Marine Biological Resource Information Appendix C1 Resource Agency Coordination Appendix C2 Marine Biological Resources Report APPENDIX C1 RESOURCE AGENCY COORDINATION 1 The ICF terrestrial biological team coordinated with relevant resource agencies to discuss 2 sensitive biological resources expected within the terrestrial biological study area (BSA). 3 A summary of agency communications and site visits is provided below. 4 California Department of Fish and Wildlife: On July 30, 2020, ICF held a conference 5 call with Greg O’Connell (Environmental Scientist) and Corianna Flannery (Environmental 6 Scientist) to discuss Project design and potential biological concerns regarding the 7 Eureka Subsea Fiber Optic Cables Project (Project). Mr. O’Connell discussed the 8 importance of considering the western bumble bee. Ms. Flannery discussed the 9 importance of the hard ocean floor substrate and asked how the cable would be secured 10 to the ocean floor to reduce or eliminate scour. The western bumble bee has been 11 evaluated in the Biological Resources section of the main document, and direct and 12 indirect impacts are avoided. The Project Description describes in detail how the cable 13 would be installed on the ocean floor, the importance of the hard bottom substrate, and 14 the need for avoidance. 15 Consultation Outcomes: 16 • The Project was designed to avoid hard bottom substrate, and RTI Infrastructure 17 (RTI) conducted surveys of the ocean floor to ensure that proper routing of the 18 cable would occur. 19 • Ms. Flannery will be copied on all communications with the National Marine 20 Fisheries Service 21 California Department of Fish and Wildlife: On August 7, 2020, ICF held a conference 22 call with Greg O’Connell to discuss a site assessment and survey approach for the 23 western bumble bee. -

Fort Ord Natural Reserve Plant List
UCSC Fort Ord Natural Reserve Plants Below is the most recently updated plant list for UCSC Fort Ord Natural Reserve. * non-native taxon ? presence in question Listed Species Information: CNPS Listed - as designated by the California Rare Plant Ranks (formerly known as CNPS Lists). More information at http://www.cnps.org/cnps/rareplants/ranking.php Cal IPC Listed - an inventory that categorizes exotic and invasive plants as High, Moderate, or Limited, reflecting the level of each species' negative ecological impact in California. More information at http://www.cal-ipc.org More information about Federal and State threatened and endangered species listings can be found at https://www.fws.gov/endangered/ (US) and http://www.dfg.ca.gov/wildlife/nongame/ t_e_spp/ (CA). FAMILY NAME SCIENTIFIC NAME COMMON NAME LISTED Ferns AZOLLACEAE - Mosquito Fern American water fern, mosquito fern, Family Azolla filiculoides ? Mosquito fern, Pacific mosquitofern DENNSTAEDTIACEAE - Bracken Hairy brackenfern, Western bracken Family Pteridium aquilinum var. pubescens fern DRYOPTERIDACEAE - Shield or California wood fern, Coastal wood wood fern family Dryopteris arguta fern, Shield fern Common horsetail rush, Common horsetail, field horsetail, Field EQUISETACEAE - Horsetail Family Equisetum arvense horsetail Equisetum telmateia ssp. braunii Giant horse tail, Giant horsetail Pentagramma triangularis ssp. PTERIDACEAE - Brake Family triangularis Gold back fern Gymnosperms CUPRESSACEAE - Cypress Family Hesperocyparis macrocarpa Monterey cypress CNPS - 1B.2, Cal IPC -

Taxonomic Key to the Saskatchewan Cryptantha Sarah Vinge-Mazer (Saskatchewan Conservation Data Centre) on Behalf of the Botanical Assessment Working Group
Taxonomic Key to the Saskatchewan Cryptantha Sarah Vinge-Mazer (Saskatchewan Conservation Data Centre) on behalf of the Botanical Assessment Working Group Introduction The Botanical Assessment Working Group has been working on placing Saskatchewan’s two perennial Cryptantha species since around 2011, following many questions and confusion from the local botanical community. The use of the name Cryptantha macounii was the source of much of the confusion, for which an explanation is now provided. The name Cryptantha macounii (=Oreocarya macounii) is a true synonym for Cryptantha celosioides (see Simpson). In Saskatchewan (and apparently Montana, see Lesica 2012) this name has instead been applied to Cryptantha spiculifera material. Furthering the confusion, the name Cryptantha nubigena has been applied to both C. spiculifera and C. celosioides in Saskatchewan and appears regularly in older taxonomic keys. The name C. macounii has to be maintained for C. spiculifera material in Sask. for now until the SKCDC can update its database with the name. C. spiculifera has been included as a synonym for C. macounii in the SKCDC vascular plant species list. This key was compiled from several sources and was written to clarify the placement of the Saskatchewan Cryptantha species and serve as interim information until the Flora of North America (FNA) on Boraginaceae is published. It is accurate to the best of our knowledge. Notes • Our two perennial species are retained in Cryptantha in this key but are expected to be moved under the genus Oreocarya in the FNA treatment (see Simpson) • The Manual of Montana Vascular Plants (Lesica 2012) provides species descriptions for all species that occur in Sask. -

-

Vascular Plants and a Brief History of the Kiowa and Rita Blanca National Grasslands
United States Department of Agriculture Vascular Plants and a Brief Forest Service Rocky Mountain History of the Kiowa and Rita Research Station General Technical Report Blanca National Grasslands RMRS-GTR-233 December 2009 Donald L. Hazlett, Michael H. Schiebout, and Paulette L. Ford Hazlett, Donald L.; Schiebout, Michael H.; and Ford, Paulette L. 2009. Vascular plants and a brief history of the Kiowa and Rita Blanca National Grasslands. Gen. Tech. Rep. RMRS- GTR-233. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station. 44 p. Abstract Administered by the USDA Forest Service, the Kiowa and Rita Blanca National Grasslands occupy 230,000 acres of public land extending from northeastern New Mexico into the panhandles of Oklahoma and Texas. A mosaic of topographic features including canyons, plateaus, rolling grasslands and outcrops supports a diverse flora. Eight hundred twenty six (826) species of vascular plant species representing 81 plant families are known to occur on or near these public lands. This report includes a history of the area; ethnobotanical information; an introductory overview of the area including its climate, geology, vegetation, habitats, fauna, and ecological history; and a plant survey and information about the rare, poisonous, and exotic species from the area. A vascular plant checklist of 816 vascular plant taxa in the appendix includes scientific and common names, habitat types, and general distribution data for each species. This list is based on extensive plant collections and available herbarium collections. Authors Donald L. Hazlett is an ethnobotanist, Director of New World Plants and People consulting, and a research associate at the Denver Botanic Gardens, Denver, CO. -

1 a B 2 A/B a 3 B 4
ATTACHMENT SS2 REGION 2 SENSITIVE SPECIES EVALUATION FORM Species: Cryptantha crassisepala (Torr. & A. Gray) Greene thick-sepal cryptantha SYNONYMS: Eritrichium crassisepalum Torr. & Gray; Krynitzkia crassisepala (Torr. & Gray) A. Gray Species comprises two varieties, both of which occur in R2: var. crassisepala var. elachantha I.M. Johnst. Criteria Rank Rationale Literature Citations var. crassisepala: se-most (Baca and Las Animas Cos) CO and sw-most (Morton and Stevens Cos) KS. A [Vouchers at KANU from Baca and Prowers Cos, CO and Morton and Stevens Cos, KS.] Note: Reports that species occurs in other KS Cos are based on misidentified specimens of C. minima, a much more wide- • Anderson 1950 spread taxon in R2 (CO, KS, NE, SD and WY, fide Freeman). It is that evaluator’s opinion that, in light of this • Clark 1996 fact, the voucher on which Hazlett reported species from Weld Co CO requires re-examination.] • Freeman in prep. 1 Status: G5T5?; KS S2. • Great Plains Flora Association 1977 Distribution Confidence in Rank High or Medium or Low • Hazlett 1998 within R2 • Kaul 1991 var. elachantha: sw ¼ (?) of CO. Weber & Wittman (2001b) cite taxon as “abundant weedy desert annual, • Weber & Wittman 2001a particularly on Mancos shale.” • Weber & Wittman 2001b Status: G5?T5? B Confidence in Rank High or Medium or Low var. crassisepala: Martin & Hutchins report taxon from ne-most and s-central counties only in NM. Other A/B published accounts (eg, Cronquist et al.; Sivinski) report that species is restricted to se-most NM and Trans- Pecos TX. However, R.L. McGregor (pers. comm.) relates that he suspects taxon occurs continuously from se-most CO and sw-most KS, s through w TX and e NM. -
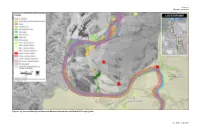
Botanical Resources and Wetlands Technical Report
Chapter 1 Affected Environment Figure 1-3g. Sensitive Biological Resources Between Shasta Dam and Red Bluff Pumping Plant 1-45 Draft – June 2013 Shasta Lake Water Resources Investigation Biological Resources Appendix – Botanical Resources and Wetlands Technical Report This page left blank intentionally. 1-46 Draft – June 2013 Chapter 1 Affected Environment Figure 1-3h. Sensitive Biological Resources Between Shasta Dam and Red Bluff Pumping Plant 1-47 Draft – June 2013 Shasta Lake Water Resources Investigation Biological Resources Appendix – Botanical Resources and Wetlands Technical Report This page left blank intentionally. 1-48 Draft – June 2013 Chapter 1 Affected Environment Figure 1-3i. Sensitive Biological Resources Between Shasta Dam and Red Bluff Pumping Plant 1-49 Draft – June 2013 Shasta Lake Water Resources Investigation Biological Resources Appendix – Botanical Resources and Wetlands Technical Report This page left blank intentionally. 1-50 Draft – June 2013 Chapter 1 Affected Environment Figure 1-3j. Sensitive Biological Resources Between Shasta Dam and Red Bluff Pumping Plant 1-51 Draft – June 2013 Shasta Lake Water Resources Investigation Biological Resources Appendix – Botanical Resources and Wetlands Technical Report This page left blank intentionally. 1-52 Draft – June 2013 Chapter 1 Affected Environment 1 Valley Oak Woodland This habitat type consists of an open savanna of 2 valley oak (Quercus lobata) trees and an annual grassland understory. Valley 3 oak is typically the only tree species present and shrubs are generally absent 4 except for occasional poison oak. Canopy cover rarely exceeds 30–40 percent in 5 valley oak woodland. This community occupies the highest portions of the 6 floodplain terrace where flooding is infrequent and shallow. -

Vascular Plants of the Coastal Dunes of Humboldt County, California
Humboldt State University Digital Commons @ Humboldt State University Botanical Studies Open Educational Resources and Data 4-2019 Vascular Plants of the Coastal Dunes of Humboldt County, California James P. Smith Jr Humboldt State University, [email protected] Follow this and additional works at: https://digitalcommons.humboldt.edu/botany_jps Part of the Botany Commons Recommended Citation Smith, James P. Jr, "Vascular Plants of the Coastal Dunes of Humboldt County, California" (2019). Botanical Studies. 41. https://digitalcommons.humboldt.edu/botany_jps/41 This Flora of Northwest California-Checklists of Local Sites is brought to you for free and open access by the Open Educational Resources and Data at Digital Commons @ Humboldt State University. It has been accepted for inclusion in Botanical Studies by an authorized administrator of Digital Commons @ Humboldt State University. For more information, please contact [email protected]. VASCULAR PLANTS OF THE COASTAL DUNES OF HUMBOLDT COUNTY, CALIFORNIA Compiled by James P. Smith, Jr. Professor Emeritus of Botany Department of Biological Sciences Humboldt State University Arcata, California Ninth Edition • August 2019 Amaryllidaceae — Onion or Amaryllis Family F E R N S Allium unifolium •One-leaved onion Dennstaedtiaceae — Bracken Fern Family Anacardiaceae — Cashew Family Pteridium aquilinum var. pubescens • Bracken fern Toxicodendron diversilobum • Poison-oak Ophioglossaceae — Adder’s-tongue Family Apocynaceae — Dogbane or Milkweed Family Botrychium multifidum • Leathery -

Boraginaceae), with an Emphasis on the Popcornflowers (Plagiobothrys)
Diversification, biogeography, and classification of Amsinckiinae (Boraginaceae), with an emphasis on the popcornflowers (Plagiobothrys) By Christopher Matthew Guilliams A dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in Integrative Biology in the Graduate Division of the University of California, Berkeley Committee in charge: Professor Bruce G. Baldwin, Chair Professor David Ackerly Professor Brent Mishler Professor Patrick O'Grady Summer 2015 Abstract Diversification, biogeography, and classification of Amsinckiinae (Boraginaceae), with an emphasis on the popcornflowers (Plagiobothrys) by Christopher Matthew Guilliams Doctor of Philosophy in Integrative Biology University of California, Berkeley Professor Bruce G. Baldwin, Chair Amsinckiinae is a diverse and ecologically important subtribe of annual herbaceous or perennial suffrutescent taxa with centers of distribution in western North America and southern South America. Taxa in the subtribe occur in all major ecosystems in California and more broadly in western North America, from the deserts of Baja California in the south where Johnstonella and Pectocarya are common, north to the ephemeral wetland ecosystems of the California Floristic Province where a majority of Plagiobothrys sect. Allocarya taxa occur, and east to the Basin and Range Province of western North America, where Cryptantha sensu stricto (s.s.) and Oreocarya are well represented. The subtribe minimally includes 9 genera: Amsinckia, Cryptantha s.s., Eremocarya, Greeneocharis, Harpagonella, Johnstonella, Oreocarya, Pectocarya, and Plagiobothrys; overall minimum-rank taxonomic diversity in the subtribe is ca. 330-342 taxa, with ca. 245--257 taxa occurring in North America, 86 in South America, and 4 in Australia. Despite their prevalence on the landscape and a history of active botanical research for well over a century, considerable research needs remain in Amsinckiinae, especially in one of the two largest genera, Plagiobothrys. -

Baja California, Mexico, and a Vegetation Map of Colonet Mesa Alan B
Aliso: A Journal of Systematic and Evolutionary Botany Volume 29 | Issue 1 Article 4 2011 Plants of the Colonet Region, Baja California, Mexico, and a Vegetation Map of Colonet Mesa Alan B. Harper Terra Peninsular, Coronado, California Sula Vanderplank Rancho Santa Ana Botanic Garden, Claremont, California Mark Dodero Recon Environmental Inc., San Diego, California Sergio Mata Terra Peninsular, Coronado, California Jorge Ochoa Long Beach City College, Long Beach, California Follow this and additional works at: http://scholarship.claremont.edu/aliso Part of the Biodiversity Commons, Botany Commons, and the Ecology and Evolutionary Biology Commons Recommended Citation Harper, Alan B.; Vanderplank, Sula; Dodero, Mark; Mata, Sergio; and Ochoa, Jorge (2011) "Plants of the Colonet Region, Baja California, Mexico, and a Vegetation Map of Colonet Mesa," Aliso: A Journal of Systematic and Evolutionary Botany: Vol. 29: Iss. 1, Article 4. Available at: http://scholarship.claremont.edu/aliso/vol29/iss1/4 Aliso, 29(1), pp. 25–42 ’ 2011, Rancho Santa Ana Botanic Garden PLANTS OF THE COLONET REGION, BAJA CALIFORNIA, MEXICO, AND A VEGETATION MAPOF COLONET MESA ALAN B. HARPER,1 SULA VANDERPLANK,2 MARK DODERO,3 SERGIO MATA,1 AND JORGE OCHOA4 1Terra Peninsular, A.C., PMB 189003, Suite 88, Coronado, California 92178, USA ([email protected]); 2Rancho Santa Ana Botanic Garden, 1500 North College Avenue, Claremont, California 91711, USA; 3Recon Environmental Inc., 1927 Fifth Avenue, San Diego, California 92101, USA; 4Long Beach City College, 1305 East Pacific Coast Highway, Long Beach, California 90806, USA ABSTRACT The Colonet region is located at the southern end of the California Floristic Province, in an area known to have the highest plant diversity in Baja California.