LOCAD-PTS: Operation of a New System for Microbial Monitoring Aboard the International Space Station (ISS)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Increment Definition and Requirements Document for Increment 15
CR 010130, Attachment A WWW.NASAWATCH.COM SSP 54015 Baseline (DRAFT) Increment Definition and Requirements Document for Increment 15 International Space Station Program Baseline September 2006 National Aeronautics and Space Administration International Space Station Program Johnson Space Center Houston, Texas SSP 54015 WWW.NASAWATCH.COM SSCN XXXX Attachment A Baseline (DRAFT) REVISION AND HISTORY PAGE REV. DESCRIPTION PUB. DATE - Initial Release (Reference per SSCD XXXXXX, EFF. XX-XX-XX) XX-XX-XX SSP 54015 WWW.NASAWATCH.COM SSCN XXXX Attachment A Baseline (DRAFT) INTERNATIONAL SPACE STATION PROGRAM INCREMENT DEFINITION AND REQUIREMENTS DOCUMENT FOR INCREMENT 15 CHANGE SHEET September, 2006 Baseline Space Station Control Board Directive XXXXXX/(X-X), dated XX-XX-XX. (X) CHANGE INSTRUCTIONS SSP 54015, Increment Definition and Requirements Document for Increment 15, has been baselined by the authority of SSCD XXXXXX. All future updates to this document will be identified on this change sheet. SSP 54015 WWW.NASAWATCH.COM SSCN XXXX Attachment A Baseline (DRAFT) INTERNATIONAL SPACE STATION PROGRAM INCREMENT DEFINITION AND REQUIREMENTS DOCUMENT FOR INCREMENT 15 Baseline (Reference SSCD XXXXXX, dated XX-XX-XX) LIST OF EFFECTIVE PAGES September, 2006 The current status of all pages in this document is as shown below: Page Change No. SSCD No. Date SSP 54015 WWW.NASAWATCH.COM SSCN XXXX Attachment A Baseline (DRAFT) INTERNATIONAL SPACE STATION PROGRAM INCREMENT DEFINITION AND REQUIREMENTS DOCUMENT FOR INCREMENT 15 SEPTEMBER 2006 i SSP 54015 WWW.NASAWATCH.COM SSCN XXXX Attachment A Baseline (DRAFT) PREFACE INCREMENT DEFINITION AND REQUIREMENTS DOCUMENT FOR INCREMENT 15 This document is the Increment Definition and Requirements Document for Increment 15. -

Space Reporter's Handbook Mission Supplement Shuttle Mission STS
CBS News Space Reporter's Handbook - Mission Supplement! Page 1 The CBS News Space Reporter's Handbook Mission Supplement Shuttle Mission STS-134/ISS-ULF6: International Space Station Assembly and Resupply Written and Produced By William G. Harwood CBS News Space Analyst [email protected] CBS News!!! 4/26/11 Page 2 ! CBS News Space Reporter's Handbook - Mission Supplement Revision History Editor's Note Mission-specific sections of the Space Reporter's Handbook are posted as flight data becomes available. Readers should check the CBS News "Space Place" web site in the weeks before a launch to download the latest edition: http://www.cbsnews.com/network/news/space/current.html DATE RELEASE NOTES 03/18/11 Initial STS-134 release 04/27/11 Updating throughout Introduction This document is an outgrowth of my original UPI Space Reporter's Handbook, prepared prior to STS-26 for United Press International and updated for several flights thereafter due to popular demand. The current version is prepared for CBS News. As with the original, the goal here is to provide useful information on U.S. and Russian space flights so reporters and producers will not be forced to rely on government or industry public affairs officers at times when it might be difficult to get timely responses. All of these data are available elsewhere, of course, but not necessarily in one place. The STS-134 version of the CBS News Space Reporter's Handbook was compiled from NASA news releases, JSC flight plans, the Shuttle Flight Data and In-Flight Anomaly List, NASA Public Affairs and the Flight Dynamics office (abort boundaries) at the Johnson Space Center in Houston. -

+ STS-123 Press
CONTENTS Section Page STS-123 MISSION OVERVIEW................................................................................................ 1 TIMELINE OVERVIEW.............................................................................................................. 11 MISSION PROFILE................................................................................................................... 15 MISSION PRIORITIES............................................................................................................. 17 MISSION PERSONNEL............................................................................................................. 19 STS-123 ENDEAVOUR CREW .................................................................................................. 21 PAYLOAD OVERVIEW .............................................................................................................. 31 KIBO OVERVIEW.................................................................................................................................. 31 KIBO MISSION CONTROL CENTER ....................................................................................................... 39 TSUKUBA SPACE CENTER.................................................................................................................... 43 SPACE STATION INTEGRATION AND PROMOTION CENTER .................................................................. 47 JAXA’S EXPERIMENTS DURING THE 1J/A STAGE................................................................................. -
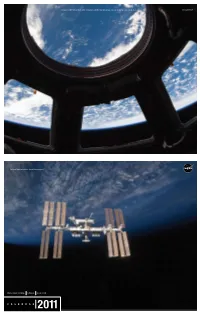
C a L E N D a R International Space Station
For more information on the International Space Station, visit: www.nasa.gov/station visit: Station, Space International the on information more For www.nasa.gov National Aeronautics and Space Administration INTERNATIONAL SPACE STATION CALENDAR 2011 A MESSAGE FROM THE PROGRAM MANAGER The International Space Station (ISS) is one of the greatest technological, geopolitical and engineering accomplishments in human 2011 history. The completion of the ISS on-orbit assembly allows for a focus on the multifaceted purpose of the ISS, one of scientific research, technology development, exploration and education. As a National Laboratory, the ISS will provide opportunities beyond NASA to academia, commercial entities and other government agencies to pursue their research and development needs in science, technology development and education. With everyone working together, we look forward to extending human presence beyond and improving life here on Earth. This calendar is designed to show all facets of the ISS using displays of astounding imagery and providing significant historical events with the hope of inspiring the next generation. NASA is appreciative of the commitment that America’s educators demonstrate each and every day as they instruct and shape the young students who will be tomorrow’s explorers and leaders. I hope you enjoy the calendar and are encouraged to learn new and exciting aspects about NASA and the ISS throughout the year. Regards, MICHAEL T. SUFFREDINI ISS Program Manager 1 2 2 3 4 6 5 LOOK HOW FAR WE’VE COME 20 JANUARY NASA has powered us into the 21st century through signature 11 accomplishments that are enduring icons of human achievement. -

STS-135: the Final Mission Dedicated to the Courageous Men and Women Who Have Devoted Their Lives to the Space Shuttle Program and the Pursuit of Space Exploration
National Aeronautics and Space Administration STS-135: The Final Mission Dedicated to the courageous men and women who have devoted their lives to the Space Shuttle Program and the pursuit of space exploration PRESS KIT/JULY 2011 www.nasa.gov 2 011 2009 2008 2007 2003 2002 2001 1999 1998 1996 1994 1992 1991 1990 1989 STS-1: The First Mission 1985 1981 CONTENTS Section Page SPACE SHUTTLE HISTORY ...................................................................................................... 1 INTRODUCTION ................................................................................................................................... 1 SPACE SHUTTLE CONCEPT AND DEVELOPMENT ................................................................................... 2 THE SPACE SHUTTLE ERA BEGINS ....................................................................................................... 7 NASA REBOUNDS INTO SPACE ............................................................................................................ 14 FROM MIR TO THE INTERNATIONAL SPACE STATION .......................................................................... 20 STATION ASSEMBLY COMPLETED AFTER COLUMBIA ........................................................................... 25 MISSION CONTROL ROSES EXPRESS THANKS, SUPPORT .................................................................... 30 SPACE SHUTTLE PROGRAM’S KEY STATISTICS (THRU STS-134) ........................................................ 32 THE ORBITER FLEET ............................................................................................................................ -
STS-135: the Final Mission Dedicated to the Courageous Men and Women Who Have Devoted Their Lives to the Space Shuttle Program and the Pursuit of Space Exploration
National Aeronautics and Space Administration STS-135: The Final Mission Dedicated to the courageous men and women who have devoted their lives to the Space Shuttle Program and the pursuit of space exploration PRESS KIT/JULY 2011 www.nasa.gov 2 011 2009 2008 2007 2003 2002 2001 1999 1998 1996 1994 1992 1991 1990 1989 STS-1: The First Mission 1985 1981 CONTENTS Section Page SPACE SHUTTLE HISTORY ...................................................................................................... 1 INTRODUCTION ................................................................................................................................... 1 SPACE SHUTTLE CONCEPT AND DEVELOPMENT ................................................................................... 2 THE SPACE SHUTTLE ERA BEGINS ....................................................................................................... 7 NASA REBOUNDS INTO SPACE ............................................................................................................ 14 FROM MIR TO THE INTERNATIONAL SPACE STATION .......................................................................... 20 STATION ASSEMBLY COMPLETED AFTER COLUMBIA ........................................................................... 25 MISSION CONTROL ROSES EXPRESS THANKS, SUPPORT .................................................................... 30 SPACE SHUTTLE PROGRAM’S KEY STATISTICS (THRU STS-134) ........................................................ 32 THE ORBITER FLEET ............................................................................................................................ -

STS-121 Press Kit
STS-121 Press Kit CONTENTS Section Page STS-116 MISSION OVERVIEW: POWER RECONFIGURATION HIGHLIGHTS STATION ASSEMBLY MISSION ...............................................................................................1 STS-116 TIMELINE OVERVIEW ...............................................................................................10 MISSION PRIORITIES.............................................................................................................12 LAUNCH AND LANDING ...........................................................................................................15 LAUNCH...............................................................................................................................................15 ABORT-TO-ORBIT (ATO)......................................................................................................................15 TRANSATLANTIC ABORT LANDING (TAL).............................................................................................15 RETURN-TO-LAUNCH-SITE (RTLS).......................................................................................................15 ABORT ONCE AROUND (AOA)...............................................................................................................15 LANDING .............................................................................................................................................15 MISSION PROFILE...................................................................................................................16 -

Research on the International Space Station: Understanding Future Potential from Current Accomplishments
https://ntrs.nasa.gov/search.jsp?R=20070016593 2019-08-30T00:40:57+00:00Z View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by NASA Technical Reports Server IAC-07-B3.4.07 RESEARCH ON THE INTERNATIONAL SPACE STATION: UNDERSTANDING FUTURE POTENTIAL FROM CURRENT ACCOMPLISHMENTS Judy M. Tate and Tracy L. Thumm Engineering and Science Contract Group, Houston, TX, U.S.A. [email protected], [email protected] Roger H. Weiss Science Applications International Corporation, Houston, TX, U.S.A. [email protected] Julie A. Robinson NASA Johnson Space Center, Houston, Texas, U.S.A. [email protected] ABSTRACT In November 2007, the International Space Station (ISS) will have supported seven years of continuous presence in space, with 15 Expeditions completed. These years have been characterized by the numerous technical challenges of assembly as well as operational and logistical challenges related to the availability of transportation by the Space Shuttle. During this period, an active set of early research objectives have also been accomplished alongside the assembly. This paper will review the research accomplishments on the ISS to date, with the objective of drawing insights on the potential of future research following completion of the ISS assembly. During the first seven years and fourteen targeted at reducing the risks to exploration Expeditions to the International Space Station missions to the Moon, Mars, and beyond [1]. (ISS), several events shaped the focus of research on the ISS and the ability of the ISS for By the end of Expedition 15 in November 2007, performing early research. -

Expedition 15 Press
TABLE OF CONTENTS Section Page Mission Overview ............................................................................................................ 1 Expedition 15 Crew ......................................................................................................... 7 Expedition 14 Spacewalks.............................................................................................. 13 Russian Soyuz TMA ........................................................................................................ 17 Soyuz Booster Rocket Characteristics ......................................................................... 23 Prelaunch Countdown Timeline ................................................................................... 24 Ascent/Insertion Timeline ............................................................................................. 25 Orbital Insertion to Docking Timeline............................................................................ 26 Key Times for Expedition 15/14 International Space Station Events............................ 31 EXPEDITION 14/ISS SOYUZ 13 (TMA-9) LANDING................................................... 33 Soyuz Entry Timeline ................................................................................................... 36 European Space Agency's Automated Transfer Vehicle Debuts ................................ 41 International Space Station: Expedition 15 Science Overview.................................... 51 The Payload Operations Center .................................................................................... -

Increment Definition and Requirements Document for Increment 18
CR 010864A, Attachment A www.nasawatch.com SSP 54018 Baseline (Draft - December 2007) Increment Definition and Requirements Document for Increment 18 International Space Station Program Baseline (Draft - December 2007) January 2008 National Aeronautics and Space Administration International Space Station Program Johnson Space Center Houston, Texas Contract Number: NNJ04AA02C CR 010864A, Attachment A www.nasawatch.com SSP 54018 Baseline (Draft - December 2007) REVISION AND HISTORY PAGE REV. DESCRIPTION PUB. DATE - Initial Release (Reference per SSCD XXXXXX, EFF. XX-XX-XX) XX-XX-XX CR 010864A, Attachment A www.nasawatch.com SSP 54018 Baseline (Draft - December 2007) PREFACE INCREMENT DEFINITION AND REQUIREMENTS DOCUMENT FOR INCREMENT 18 This document is the Increment Definition and Requirements Document for Increment 18. Official delivery of this document is under control of the Space Station Control Board (SSCB). Any changes or revisions will be jointly agreed to and signed by the National Aeronautics and Space Administration (NASA) and the affected International Partners (IPs). i CR 010864A, Attachment A www.nasawatch.com SSP 54018 Baseline (Draft - December 2007) NASA/ROSCOSMOS INTERNATIONAL SPACE STATION PROGRAM INCREMENT DEFINITION AND REQUIREMENTS DOCUMENT FOR INCREMENT 18 JANUARY 2008 Michael T. Suffredini Alexi Krasnov National Aeronautics and Space Roscosmos Administration Director, Manned Flight Program Manager, International Space Station Program Date Date ii CR 010864A, Attachment A www.nasawatch.com SSP 54018 Baseline (Draft - December 2007) NASA/CSA INTERNATIONAL SPACE STATION PROGRAM INCREMENT DEFINITION AND REQUIREMENTS DOCUMENT FOR INCREMENT 18 JANUARY 2008 Michael T. Suffredini Benoit Marcotte National Aeronautics and Space CSA Program Manager Administration Manager, International Space Station Program Date Date iii CR 010864A, Attachment A www.nasawatch.com SSP 54018 Baseline (Draft - December 2007) NASA/ESA INTERNATIONAL SPACE STATION PROGRAM INCREMENT DEFINITION AND REQUIREMENTS DOCUMENT FOR INCREMENT 18 JANUARY 2008 Michael T. -

Expedition 16 Press
TABLE OF CONTENTS Section Page MISSION OVERVIEW............................................................................................................... 1 EXPEDITION 16 CREW ............................................................................................................ 7 MISSION MILESTONES ........................................................................................................... 17 EXPEDITION 16 SPACEWALKS................................................................................................ 21 RUSSIAN SOYUZ TMA ............................................................................................................. 25 SOYUZ BOOSTER ROCKET CHARACTERISTICS .................................................................................... 31 PRELAUNCH COUNTDOWN TIMELINE................................................................................................... 32 ASCENT/INSERTION TIMELINE............................................................................................................ 33 ORBITAL INSERTION TO DOCKING TIMELINE ...................................................................................... 34 KEY TIMES FOR EXPEDITION 16/15 INTERNATIONAL SPACE STATION EVENTS ................................... 39 EXPEDITION 16/SOYUZ TMA-10 LANDING ........................................................................................... 41 SOYUZ ENTRY TIMELINE ..................................................................................................................... -

Increment Definition and Requirements Document for Increment 14
WWW.NASAWATCH.COM SSP 54014 Revision A Increment Definition and Requirements Document for Increment 14 International Space Station Program Revision A April 2006 National Aeronautics and Space Administration International Space Station Program Johnson Space Center Houston, Texas Contract Number: NNJ04AA02C SSP 54014 WWW.NASAWATCH.COM Revision A REVISION AND HISTORY PAGE REV. DESCRIPTION PUB. DATE - Initial Release (Reference per SSCD 009752, EFF. 02-14-06) Early Release 03-10-06 Program Release XX-XX-XX A Revision A (Reference per SSCD XXXXXX, EFF. XX-XX-XX) XX-XX-XX SSP 54014 WWW.NASAWATCH.COM Revision A INTERNATIONAL SPACE STATION PROGRAM INCREMENT DEFINITION AND REQUIREMENTS DOCUMENT FOR INCREMENT 14 CHANGE SHEET Month XX, XXXX Revision A Space Station Control Board Directive No. XXXXXX/(X-X), dated XX-XX-XX. (X) CHANGE INSTRUCTIONS SSP 54014, Increment Definition and Requirements Document for Increment 14, has been approved by the authority of SSCD XXXXXX. All future updates to this document will be identified on this change sheet. SSP 54014 WWW.NASAWATCH.COM Revision A INTERNATIONAL SPACE STATION PROGRAM INCREMENT DEFINITION AND REQUIREMENTS DOCUMENT FOR INCREMENT 14 Revision A (Reference SSCD XXXXXX, dated XX-XX-XX) LIST OF EFFECTIVE PAGES Month XX, XXXX The current status of all pages in this document is as shown below: Page Change No. SSCD No. Date i - xii Revision A XXXXXX Month XX, XXXX 1-1 - 1-2 Revision A XXXXXX Month XX, XXXX 2-1 - 2-3 Revision A XXXXXX Month XX, XXXX 3-1 - 3-8 Revision A XXXXXX Month XX, XXXX 4-1 - 4-10 Revision