Medical Laboratory Assistant, a Suggested Guide for A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

VIROLOGY for MEDICAL LABORATORY STUDENTS Mwamisi, Muthwii, Mwala, Mokua
VIROLOGY for MEDICAL LABORATORY STUDENTS Mwamisi, Muthwii, Mwala, Mokua To the Reader Virology is an important subject in Medical Laboratory Sciences. Medical Laboratory Medicine puts a technologist/technician in the fore front in diagnosis and proper interpretation of the correct diseases by a clinician. Research on viruses, therefore, has gained a lot of momentum in recent years due to the Medical importance they contribute to the field of medicine. A lot of this work goes through the medical laboratory Department where it is handled by the medical laboratory students, especially, on practical experience. The purpose of this manual is, therefore, to provide to the student adequate up-to-date information in preparation for the work that the student will perform in any Medical Laboratory Department, whether research or routine work. We hope this information will not only be useful to the medical laboratory Students or staff, but!also will be a reference material for others in Health or health related field. Joseph M. Mwamisi, PhD Samson M. Muthwii PhD Denis M. Mwala M.Se. John M. Mokua M.Se., ) 2 TABLE OF CONTENTS 1. VIROLOGY 6 1.1 Definition of Virology 6 1.2 Definition of Viruses 6 1.3 Differences between Bacteria and Viruses 7 1.4 Morphology of Viruses 7 2. CLASSIFICATION OF VIRUSES 9 2.1 Classification 9 Epidemiological Analysis 9 Physical and Chemical Properties Analysis 9 DNA Viruses 9 RNA Viruses 9 Enteric Viruses 10 Respiratory Viruses ' 10 Arthropod-Borne Viruses (Arboviruses) 10 2.2. DNA Viruses 10 Poxvirusae 10 Ridoviridae 10 2.3. RNA Viruses 10 Picornaviridae 11 3. -

Infection Control in Dentistry: How to Asepsis Photographic Mirrors?
Infection control in dentistry: how to asepsis photographic mirrors? Amanda Osório Ayres de Freitas* Mariana Marquezan* Giselle Naback Lemes Vilani* Rodrigo César Santiago* Luiz Felipe de Miranda Costa* Sandra Regina Torres** Abstract: The aim of this study was to evaluate the efficacy of six different methods of disinfection and sterilization of intraoral photographic mirrors through microbiological testing and to analysis their potential harm to mirrors’ surface. Fourteen occlusal mirrors were divided into seven groups. Group 1 comprised two mirrors as received from manufacturer. The other six groups comprised mirrors disinfected/sterilized by autoclave, immersion in enzymatic detergent, and friction with chlorhexidine detergent, chlorhexidine wipes, common detergent and 70% ethylic alcohol. Microbiological and quality surface analyses were performed. Sterilization in autoclave was microbiologic effective, but caused damage to the mirror surface. Chlorhexidine (in wipes or detergent) and liquid soap were effective disinfectant agents for photographic mirrors decontamination, without harmful effect on its surface. Enzymatic detergent immersion and friction with 70% ethylic alcohol were not effective as disinfectant agents for photographic mirrors decontamination. According to the results, the more effective and safe methods for photographic mirrors disinfection were friction with chlorhexidine wipes or detergent, as well as liquid soap. Results, the most efficacious methods for photographic mirrors disinfection were friction with chlorhexidine wipes and detergent, as well as common detergent. Descriptors: Dental Instruments; Decontamination; Microbiology; Surface Properties. *Doutoranda em Odontologia na Universidade Federal do Rio de Janeiro (UFRJ), Rio de Janeiro, RJ, Brasil **Pósdoutora em odontologia pela University of Washington (UW), Seattle, WA, Estados Unidos ISSN 22365843 │ 93 Introduction Dental photography is an important tool for diagnostic and treatment planning, and it’s also a registration of the patient’s condition before and after treatment. -

Medical Laboratory Scientist LIMITED ENTRY BACHELOR of APPLIED SCIENCE (BAS) REQUIRED CREDITS: 120 DEGREE CODE: MLS-BAS
MEDICAL LABORATORY PROGRAM Medical Laboratory Scientist LIMITED ENTRY BACHELOR OF APPLIED SCIENCE (BAS) REQUIRED CREDITS: 120 DEGREE CODE: MLS-BAS This is a limited-entry program. Some of these courses are program prerequisites and MUST be completed before a student is considered eligible for entry into the Program. Students MUST attend a Health Programs orientation and meet with a Health Programs advisor for additional advisement. DESCRIPTION The Medical Laboratory Scientist (MLS) is an important member of the health care team in hospitals, clinics, medical research and teaching centers, and is an indispensable participant with physicians in providing critical diagnostic information. The MLS functions as a dependable, ambitious and highly motivated professional capable of handling high stress situations with ease and confidence. The Medical Laboratory Scientist performs and interprets diagnostic laboratory procedures using state-of-the-art instrumentation to aid in the detection, diagnosis, and treatment of disease; monitors the standards of accuracy and precision in the performance of tests; performs routine maintenance; analyzes and corrects instrument problems; researches, evaluates, and implements new procedures; and may be responsible for fiscal/personnel management of laboratory. The Bachelor of Applied Science degree in Medical Laboratory Scientist combines academic and laboratory courses on campus with practical experience at clinical affiliate sites. The program is accredited by the National Accrediting Agency for Clinical Laboratory Sciences (NAACLS), 5600 N. River Road, Suite 720, Rosemont, IL 60018-5119, (877) 939-3597. Students successfully completing the program are eligible to take a national certifying examination. STUDENT LEARNING OUTCOMES • Select appropriate courses of action in accordance with established laboratory procedures. -

Scheme for Affiliation of Private Hospitality Institutes Offering Programs Related to Hospitality Education & Training
SCHEME FOR AFFILIATION OF PRIVATE HOSPITALITY INSTITUTES OFFERING PROGRAMS RELATED TO HOSPITALITY EDUCATION & TRAINING NATIONAL COUNCIL FOR HOTEL MANAGEMENT & CATERING TECHNOLOGY (NCHMCT) A-34, Sector 62, NOIDA – 201 309 (UP) SCHEME FOR AFFILIATION OF HOSPITALITY PRIVATE INSTITUTES OFFERING PROGRAMS RELATED TO HOSPITALITY EDUCATION AND TRAINING NEED FOR PRIVATE SECTOR PARTICIPATION: The training capacity available in the Government Institutes of Hotel Management/Food Craft Institutes is being augmented continually to maximize the delivery of trained personnel to the industry. The demand of trained manpower leaves enough scope for delivery of quality trained manpower by the institutes set up in private sector. It has therefore become necessary that existing private institutions imparting hospitality education and training assume a significant role in meeting the expanding demand for quality trained personnel. In this context, participation of educational institutes in the private sector through scheme of affiliation with NCHMCT has been launched to bridge gap between demand and supply of quality professionals to the expanding Industry. Existing hospitality programs ranging from Certificate level to Degree level offered by National Council for Hotel Management has enabled candidates from private institutes qualify for a proficiency that is in line with the quality of students turned out by the best of affiliated Institutes in the government sector. The scheme of affiliation launched in year 2006 culminated in affiliation of 4 private Institutes. Subsequently, Council invited applications from private Institutes and affiliated 10 more Institutes of Hotel Management in private sector. As on date, total 14 private Institutes are affiliated to this Council. Original scheme of affiliation has been revised in present form which brings out clarity in requirements and assessment thereof by experts Committee in a transparent manner. -

ANTT Guidelines
www.antt.org ANTT Guidelines The ANTT Clinical Guideline for the Preparation & Administration of Peripheral and Central Intravenous Medications (IV Therapy) Rationale and supporting evidence ANTT IV Prep and Administration V3 .0 2013 The Association for Safe Aseptic Technique (ASAP) www.antt.org www.antt .org ® © 2013 Aseptic Non Touch Technique (ANTT) This document is a publication of The-ASAP and all rights of copyright, intellectual property and Trademark are reserved. ANTT is protected to prevent dilution and misrepresentation of the practice framework so as to avoid ANTT becoming another unhelpful generic term for aseptic technique that is variably interpreted. For guidance see [email protected]. This document may however, be freely reviewed, copied and translated, in part, or in whole, for LOCAL, SINGLE ORGANIZATION educational use. It must not be published via the www/internet or its content used for production and publication of dedicated ANTT e-learning resources. ANTT is not for sale or for use in conjunction with commercial purposes. The-ASAP provide a number of free core ANTT resources to help disseminate and train healthcare staff. The- ASAP requests that the balance it determines between free dissemination and protection of the standard is respected in the interests of patient safety. Disclaimer: The-ASAP provides the ANTT Clinical Practice Framework and ANTT Clinical Guidelines to healthcare organizations in good faith in a collaboration to promote effective aseptic technique. It is the responsibility of healthcare organizations to implement ANTT effectively. No guarantee or responsibility for the application or outcome of clinical practice can be, or is, assumed or accepted by The-ASAP/ANTT. -

Module 6: Principles of Asepsis
Module 6: Medical and Surgical Asepsis Module 6: Medical and Surgical Asepsis Minimum Number of Theory Hours: 2 Suggested Theory Hours: 5 Recommended Clinical Hours: 8 Statement of Purpose: The purpose of this unit is to present information about asepsis and the control of infection. Procedures and precautions to protect patient/patients/residents, health care workers and others from infection are presented, including standard precautions, transmission- based precautions and biohazardous waste management. Terminology 1. Acquired Immunodeficiency 21. Escherichia coli (E. coli) 40. Non-intact Syndrome (AIDS) 22. Excretions 41. Nosocomial 2. Airborne precautions 23. Exposure incident 42. Occupational Safety and Health 3. Asepsis 24. Flora Administration (OSHA) 4. Athlete’s foot 25. Fungus 43. Pathogens 5. Bacteria 26. Health Care-Associated Infection 44. Personal Protective Equipment 6. Barriers (HAI) (PPE) 7. Biohazard symbol 27. Hepatitis A, B, C, D, E 45. Pneumonia 8. Bloodborne 28. Herpes zoster 46. Precautions 9. Carrier spore 29. Host 47. Protozoa 10. Centers for Disease Control (CDC) 30. Immunity 48. Reservoir 11. Chain of infection 31. Infection 49. Reverse isolation 12. Communicable 32. Infectious agent 50. Rickettsia 13. Contact precautions 33. Influenza 51. Scabies 14. Contagious microbes 34. Isolation 52. Sepsis 15. Contamination 35. Lice 53. Standard precautions 16. Disinfection 36. Material Safety Data Sheet (MSDS) 54. Sterilization 17. Disorientation 37. Methicillin-Resistant 55. Streptococcus 18. Disposable Staphylococcus -

The Efficacy of Three Hand Asepsis Techniques Using Chlorhexidine Gluconate (Chg 2%)
A RTIGO Eficácia de três métodos de degermação das mãos utilizando gluconato de clorexidina O degermante (GCH 2%) RIGINAL THE EFFICACY OF THREE HAND ASEPSIS TECHNIQUES USING CHLORHEXIDINE GLUCONATE (CHG 2%) EFICACIA DE TRES MÉTODOS DE DESINFECCIÓN DE LAS MANOS UTILIZANDO GLUCONATO DE CLORHEXIDINA ANTISÉPTICA (GHC 2%) Érika Rossetto da Cunha1, Fabiana Gonçalves de Oliveira Azevedo Matos2, Adriana Maria da Silva3, Eutália Aparecida Cândido de Araújo4, Karine Azevedo São Leão Ferreira5, Kazuko Uchikawa Graziano6 RESUMO ABSTRACT RESUMEN A degermação cirúrgica das mãos e dos The scrubbing of hands and forearms using La desinfección quirúrgica de manos y an- antebraços é um procedimento que inte- anti septi c agents has been the standard pre- tebrazos es un procedimiento que integra gra as ati vidades de paramentação cirúr- operati ve procedure to prevent surgical site las acti vidades prequirúrgicas como me- gica como uma medida de prevenção de infecti on. With the introducti on of anti sep- dida de prevención contra infección del infecção do síti o cirúrgico. Com o advento ti c agents, the need to use brushes for pre- siti o quirúrgico. Con el advenimiento de la dos princípios anti ssépti cos degermantes, operati ve disinfecti on has been questi oned anti sepsia desinfectante, se cuesti ona y se a necessidade do uso de escovas para a and it has been recommended that the pro- recomienda dejar de lado el uso de cepillos degermação cirúrgica tem sido questi ona- cedure be abandoned due to the injuries it debido a lesiones provocadas en piel. Para da e recomendado o abandono deste uso may cause to the skin. -

To Eritrea Health and Population Project March 12-28, 1997
PROCUREMENT ASSISTANCE TO ERITREA HEALTH AND POPULATION PROJECT MARCH 12-28, 1997 Asmara, Entrea - Todd DIckens I I I BASICS ActlVlty Code OI7-ER-OI-055 USAID Contract No HRN-Q-17-93-00032-00 I I TABLE OF CONTENTS ACKNOWLEDGMENTS v ACRONYMS VB EXECUTIVE SUMMARY 1 PURPOSE OF VISIT 4 BACKGROUND 5 TRIP ACTIVITIES 6 EvaluatIOn of Phannacor' s Request for ChemIcal Reagents Pncmg 7 EvaluatIOn of Phanna cor's Request for Glassware and SupplIes Pncmg 9 EvaluatIOn of Phanna cor's Request for Laboratory EqUIpment Pncmg 12 ComplIance WIth USAIDIBASICS Procurement ReqUIrements 15 I Issue of Updated Requests for Quote 16 ReVIew of DocumentatIOn ReqUIrements for Purchase Orders 16 I ReVIew of ReqUIrements for Momtonng and ReceIVmg Slnpments 17 I RESULTS AND CONCLUSIONS 17 POST-TRIP PROJECT ACTIVITIES 18 I APPENDIXES AppendIX A List of Contacts, Entrea, March 1997 AppendIxB PATH Scope of Work I AppendIx C Phannacor ChemIcal BId AnalysIs Sheets AppendIx D Phannacor Glassware Quote AnalYSIS Sheets AppendIx E Phannacor Instrument BId AnalysIs Sheet I AppendIx F Phannacor Laboratory Instrument Request for Quote, March 22, 1997 AppendIx G Proposed General OutlIne ofNDQCL Procurement Process, March 18, 1997 I AppendIX H Entrea NDQCL Purchase Order DocumentatIOn ReqUIrements, March 27, 1997 AppendIX I Entrea NDQCL Proposed Purchase Order Trackmg, Customs Clearmg, I DelIvery, and Payment ResponSIbIlItIes, March 27, 1997 AppendIX J Drug QUalIty Control Laboratory EqUIpment and SupplIes ReceIpt by Phannacor and Entrean Mlmstry of Health I AppendIX K Entrea NDQCL -
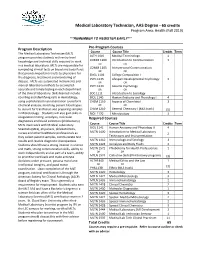
Medical Laboratory Technician 65 Credit AAS Program Guide
Medical Laboratory Technician, AAS Degree - 65 credits Program Area: Health (Fall 2019) ***REMEMBER TO REGISTER EARLY*** Program Description Pre-Program Courses Course Course Title Credits Term The Medical Laboratory Technician (MLT) program provides students with entry level ALTH 1410 Medical Terminology 1 knowledge and technical skills required to work COMM 1100 Introduction to Communication 3 in a medical laboratory. MLTs are responsible for OR OR COMM 1105 Interpersonal Communication completing clinical tests on blood and body fluids OR OR that provide important results to physicians for ENGL 1106 College Composition I the diagnosis, treatment and monitoring of PSYC 1135 Lifespan Developmental Psychology 3 disease. MLTs use automated instruments and OR OR manual laboratory methods to accomplish PSYC 1120 General Psychology accurate and timely testing in each department OR OR of the clinical laboratory. Skills learned include SOC 1111 Introduction to Sociology counting and identifying cells in Hematology, BIOL 1140 Human Anatomy and Physiology I 4 using sophisticated instrumentation to perform CHEM 1110 Aspects of Chemistry I 3 chemical analysis, matching patient blood types OR OR to donors for transfusion and preparing samples CHEM 1210 General Chemistry I (MLS track) (5) in Microbiology. Students will also gain skills in BIOL 1170 Microbiology 3 coagulation testing, urinalysis, molecular Required Courses diagnostics and blood collection (phlebotomy). MLTs must work with Medical Laboratory Course Course Title Credits Term Scientists (MLS), physicians, phlebotomists, BIOL 1141 Human Anatomy and Physiology II 4 MLTN 1400 Introduction to Medical Laboratory nurses and other healthcare professionals as 2 they collect patient samples, communicate test Techniques and Instrumentation results and resolve diagnostic discrepancies. -

Summary ANTIMICROBIAL AGENTS
ANTIMICROBIAL AGENTS Summary of a Round Table Discussion By Mark H. Lepper, M.D., and Harris D. Riley, Jr., M.D. Department of Preventive Medicine, University of Illinois (M.H.L.), and Department of Pediatrics, University of Oklahoma (H.D.R.) NTIMKROBIAL agents have had an espe- sistance of the host to infection. (The effect cially great impact in pediatrics. Al- of cortisone on stneptococcal infections in though many diseases have been conquered the rabbit was cited : 58 out of 66 rabbits easily with proper antimicrobial therapy, pnetreated with cortisone died; 5 of 60 con- there still remain difficulties and failures. trol animals died.) Instances of empyema Dr. Leppen opened the session with a dis- developing during treatment of pneuimo- cussion of some of the failures of antimi- coccal pneumonia with both antibiotics and crobial therapy. ACTH were described. The discussants agreed that at no time should adrenal con- HYPERACUTE INFECTIONS ticosteroids be used in the treatment of in- Hyperacute infections, i.e., infections fectious processes without simultaneoums ad- which are often fatal within 24 hours from ministration of adequate amounts of appro- the onset of symptoms, and resistant strains pniate antibiotics. of organisms, account for the vast majority Dr. Lepper reviewed some of the salient of failures in the use of antibiotics. A few facts in connection with the use of cortisone cases cannot be classified into either cate- and allied substances in the treatment of gory and remain as unexplained failures. overwhelming infections, including menin- The magnitude of the problem of hypen- gococcemia and the Waterhouse-Fnidenich- acute infections can be judged by the fact sen syndrome. -

Laboratory Medicine: a National Status Report
Laboratory Medicine: A National Status Report Prepared for: Division of Laboratory Systems National Center for Preparedness, Detection, and Control of Infectious Diseases Centers for Disease Control and Prevention Prepared by: The Lewin Group Under subcontract to Battelle Memorial Institute May 2008 Laboratory Medicine: A National Status Report Prepared for: Division of Laboratory Systems National Center for Preparedness, Detection, and Control of Infectious Diseases Centers for Disease Control and Prevention Prepared by: The Lewin Group Julie Wolcott, MA Amanda Schwartz Clifford Goodman, PhD May 2008 Laboratory Medicine: A National Status Report Executive Summary TABLE OF CONTENTS ACKNOWLEDGMENTS v EXECUTIVE SUMMARY 1 The Value of Laboratory Medicine to Health Care ....................................................................... 2 Market Profile of the Laboratory Medicine Sector ........................................................................ 3 Laboratory Medicine Workforce...................................................................................................... 4 Quality and the Total Testing Process............................................................................................. 4 Quality Systems and Performance Measurement ......................................................................... 6 Laboratory Information Systems ..................................................................................................... 6 Federal Regulatory Oversight Of Laboratory Medicine.............................................................. -

El Paso Community College Syllabus Part II Official Course Description
MLAB 2434; Revised Fall 2019/Spring 2020 El Paso Community College Syllabus Part II Official Course Description SUBJECT AREA Medical Laboratory Technology COURSE RUBRIC AND NUMBER MLAB 2434 COURSE TITLE Clinical Microbiology COURSE CREDIT HOURS 4 3 : 4 Credits Lec Lab I. Catalog Description Provides instruction in the theory, practical application, and pathogenesis of clinical microbiology, including collection, quality control, quality assurance, lab safety, setup, identification, susceptibility testing, and reporting results. A grade of "C" or better is required in this course to take the next course. Corequisite: MLAB 2360. (3:4). Lab fee. II. Course Objectives A. Unit I. Introduction to Medical Microbiology 1. State general safety rules for handling pathogenic cultures and potentially infective clinical specimens, to include: a. the importance of personal cleanliness, frequent handwashing, and not putting pens and pencils in the mouth. b. the importance of wearing a lab coat to protect clothing from contamination. c. the importance of keeping personal articles such as books, coats, handbags, etc., off of bench areas. d. reporting all spills of infectious materials and the proper method of cleaning up infectious spills. e. reporting all spills of hazardous materials and the proper method of cleaning up hazardous spills. f. the importance of not eating, drinking, smoking, or applying cosmetics in the laboratory. g. the importance of not wearing open sandals in the laboratory. h. the importance of proper trash disposal--separating paper trash from infectious materials and disposing of infectious materials only in specially marked biohazard bags and paper trash in the regular trash cans. i. reporting any cut or accident involving infectious material, no matter how small.