Threatened Tasmanian Ferns
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

LOCAL PROVISIONS SCHEDULE SUPPORTING REPORT April 2019
LOCAL PROVISIONS SCHEDULE SUPPORTING REPORT April 2019 Cover photograph: Coastal Vegetation Killiecrankie Bay Flinders Local Provisions Schedule Supporting Report Page 2 of 201 Agenda Attachments-Draft LPS (Tasmanian Planning Scheme) Contents 1.0 Introduction………………………………………………………………………………………6 2.0 LPS Criteria –LUPAA Section 34…………………………………………………………………..8 2.1 State Planning Provisions………………………………………………………………..8 2.2 Contents of LPSs-Section 32 of LUPAA…………………………………………………..9 2.2.1 Municipal Area……………………………………………………………...9 2.2.2 Mandatory requirements…………………………………………………..9 2.2.3 Spatial Application of the State Planning Provisions……………………… 9 2.2.4 Matters a Planning scheme may or may not regulate……………………10 2.2.5 Use of Overlays and Lists………………………………………………… 10 2.2.6 Land Reserved for Public Purposes……………………………………… 10 2.2.7 Application of the Detail of the SPP to a Particular Place……………… 10 2.2.8 Overriding Provisions…………………………………………………… 10 2.2.9 Modification of Application of SPPs……………………………………….11 2.2.10 Limitations of LPS………………………………………………………… 11 2.2.11 LPS may include……………………………………………………………11 2.3 LUPAA Schedule 1 Objectives………………………………………………………… 12 2.4 State Policies…………………………………………………………………………… 19 2.4.1 Tasmanian State Coastal Policy 1996………………………………………19 2.4.2 State Policy on the Protection of Agricultural Land 2009………………….22 2.4.3 State Policy on Water Quality Management 1997……………………… 23 2.4.4 National Environment Protection Measures……………………………….24 2.5 Northern Tasmania Regional Land Use Strategy……………………………………… 24 2.6 Flinders Council -

<I> Salpichlaena</I>
Blumea 64, 2019: 1–22 www.ingentaconnect.com/content/nhn/blumea RESEARCH ARTICLE https://doi.org/10.3767/blumea.2018.64.01.01 Taxonomy and evolutionary history of the neotropical fern genus Salpichlaena (Blechnaceae) G.G. Cárdenas1,*, S. Lehtonen2, H. Tuomisto1 Key words Abstract Salpichlaena is a distinctive fern genus characterised by 2-pinnate climbing fronds with indeterminate growth. The number of species in the genus has been a matter of debate. Taxonomic studies are made difficult by ferns within-frond variability in pinna morphology and size, and by herbarium material being incomplete. We systematically hybrid documented 62 morphological traits in 283 herbarium specimens and sequenced 52 Salpichlaena and 11 outgroup Neotropics specimens. DNA sequences included plastid genes (rbcL, rpoC1 and rps4), intergenic spacers (rps4-trnS, trnH- phylogeny psbA and trnG-trnR) and a nuclear gene (pgiC). Phylogenetic analyses based on the plastid markers divided the Salpichlaena samples into six major clades. We recognise the three deepest clades as distinct species (S. hookeriana, S. papyrus systematics sp. nov. and S. volubilis), and each of the four shallower clades as a subspecies of S. volubilis. Furthermore, we taxonomy suggest that a group of specimens, placed into different clades in the plastid and nuclear trees and showing mixed morphological characters, represent a fourth species of hybrid origin (S. hybrida sp. nov.). The most important di- agnostic characters are: degree of lamina reduction in fertile pinnules; pinna/pinnule apex incisions, pinna/pinnule margin thickness and lamina texture in sterile pinna/pinnules; presence or absence of foliar buds; shape of scales; and the appearance of the abaxial surface of the lamina (uniform or with stomata on small white protuberances). -

Download Document
African countries and neighbouring islands covered by the Synopsis. S T R E L I T Z I A 23 Synopsis of the Lycopodiophyta and Pteridophyta of Africa, Madagascar and neighbouring islands by J.P. Roux Pretoria 2009 S T R E L I T Z I A This series has replaced Memoirs of the Botanical Survey of South Africa and Annals of the Kirstenbosch Botanic Gardens which SANBI inherited from its predecessor organisations. The plant genus Strelitzia occurs naturally in the eastern parts of southern Africa. It comprises three arborescent species, known as wild bananas, and two acaulescent species, known as crane flowers or bird-of-paradise flowers. The logo of the South African National Biodiversity Institute is based on the striking inflorescence of Strelitzia reginae, a native of the Eastern Cape and KwaZulu-Natal that has become a garden favourite worldwide. It sym- bolises the commitment of the Institute to champion the exploration, conservation, sustain- able use, appreciation and enjoyment of South Africa’s exceptionally rich biodiversity for all people. J.P. Roux South African National Biodiversity Institute, Compton Herbarium, Cape Town SCIENTIFIC EDITOR: Gerrit Germishuizen TECHNICAL EDITOR: Emsie du Plessis DESIGN & LAYOUT: Elizma Fouché COVER DESIGN: Elizma Fouché, incorporating Blechnum palmiforme on Gough Island PHOTOGRAPHS J.P. Roux Citing this publication ROUX, J.P. 2009. Synopsis of the Lycopodiophyta and Pteridophyta of Africa, Madagascar and neighbouring islands. Strelitzia 23. South African National Biodiversity Institute, Pretoria. ISBN: 978-1-919976-48-8 © Published by: South African National Biodiversity Institute. Obtainable from: SANBI Bookshop, Private Bag X101, Pretoria, 0001 South Africa. -
Gauging Station Index
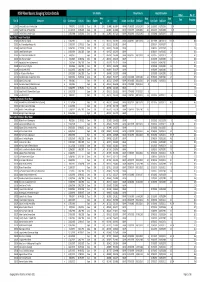
Site Details Flow/Volume Height/Elevation NSW River Basins: Gauging Station Details Other No. of Area Data Data Site ID Sitename Cat Commence Ceased Status Owner Lat Long Datum Start Date End Date Start Date End Date Data Gaugings (km2) (Years) (Years) 1102001 Homestead Creek at Fowlers Gap C 7/08/1972 31/05/2003 Closed DWR 19.9 -31.0848 141.6974 GDA94 07/08/1972 16/12/1995 23.4 01/01/1972 01/01/1996 24 Rn 1102002 Frieslich Creek at Frieslich Dam C 21/10/1976 31/05/2003 Closed DWR 8 -31.0660 141.6690 GDA94 19/03/1977 31/05/2003 26.2 01/01/1977 01/01/2004 27 Rn 1102003 Fowlers Creek at Fowlers Gap C 13/05/1980 31/05/2003 Closed DWR 384 -31.0856 141.7131 GDA94 28/02/1992 07/12/1992 0.8 01/05/1980 01/01/1993 12.7 Basin 201: Tweed River Basin 201001 Oxley River at Eungella A 21/05/1947 Open DWR 213 -28.3537 153.2931 GDA94 03/03/1957 08/11/2010 53.7 30/12/1899 08/11/2010 110.9 Rn 388 201002 Rous River at Boat Harbour No.1 C 27/05/1947 31/07/1957 Closed DWR 124 -28.3151 153.3511 GDA94 01/05/1947 01/04/1957 9.9 48 201003 Tweed River at Braeside C 20/08/1951 31/12/1968 Closed DWR 298 -28.3960 153.3369 GDA94 01/08/1951 01/01/1969 17.4 126 201004 Tweed River at Kunghur C 14/05/1954 2/06/1982 Closed DWR 49 -28.4702 153.2547 GDA94 01/08/1954 01/07/1982 27.9 196 201005 Rous River at Boat Harbour No.3 A 3/04/1957 Open DWR 111 -28.3096 153.3360 GDA94 03/04/1957 08/11/2010 53.6 01/01/1957 01/01/2010 53 261 201006 Oxley River at Tyalgum C 5/05/1969 12/08/1982 Closed DWR 153 -28.3526 153.2245 GDA94 01/06/1969 01/09/1982 13.3 108 201007 Hopping Dick Creek -

Vegetation Benchmarks Rainforest and Related Scrub
Vegetation Benchmarks Rainforest and related scrub Eucryphia lucida Vegetation Condition Benchmarks version 1 Rainforest and Related Scrub RPW Athrotaxis cupressoides open woodland: Sphagnum peatland facies Community Description: Athrotaxis cupressoides (5–8 m) forms small woodland patches or appears as copses and scattered small trees. On the Central Plateau (and other dolerite areas such as Mount Field), broad poorly– drained valleys and small glacial depressions may contain scattered A. cupressoides trees and copses over Sphagnum cristatum bogs. In the treeless gaps, Sphagnum cristatum is usually overgrown by a combination of any of Richea scoparia, R. gunnii, Baloskion australe, Epacris gunnii and Gleichenia alpina. This is one of three benchmarks available for assessing the condition of RPW. This is the appropriate benchmark to use in assessing the condition of the Sphagnum facies of the listed Athrotaxis cupressoides open woodland community (Schedule 3A, Nature Conservation Act 2002). Benchmarks: Length Component Cover % Height (m) DBH (cm) #/ha (m)/0.1 ha Canopy 10% - - - Large Trees - 6 20 5 Organic Litter 10% - Logs ≥ 10 - 2 Large Logs ≥ 10 Recruitment Continuous Understorey Life Forms LF code # Spp Cover % Immature tree IT 1 1 Medium shrub/small shrub S 3 30 Medium sedge/rush/sagg/lily MSR 2 10 Ground fern GF 1 1 Mosses and Lichens ML 1 70 Total 5 8 Last reviewed – 2 November 2016 Tasmanian Vegetation Monitoring and Mapping Program Department of Primary Industries, Parks, Water and Environment http://www.dpipwe.tas.gov.au/tasveg RPW Athrotaxis cupressoides open woodland: Sphagnum facies Species lists: Canopy Tree Species Common Name Notes Athrotaxis cupressoides pencil pine Present as a sparse canopy Typical Understorey Species * Common Name LF Code Epacris gunnii coral heath S Richea scoparia scoparia S Richea gunnii bog candleheath S Astelia alpina pineapple grass MSR Baloskion australe southern cordrush MSR Gleichenia alpina dwarf coralfern GF Sphagnum cristatum sphagnum ML *This list is provided as a guide only. -

Convict Trail
CONVICT TRAIL From historic Richmond to the Tasman START: Hobart DURATION: 1 - 3 days National Park, Eaglehawk Neck and NATIONAL PARKS ON THIS ROUTE: Port Arthur Historic Site, this fascinating > Tasman National Park journey is rich in convict history and natural beauty. The Tasman Peninsula is a place of breathtaking seascapes, some of the tallest sea cliffs in the world, and wild ocean views. LEG TIME / DISTANCE Hobart to Richmond 25 min / 27 km Richmond to Port Arthur 1 hr 10 min / 83 km Port Arthur to Hobart 1 hr 20 min / 95 km Hobart - Richmond > Drive to the village of Richmond, with its colonial past, antique shops, art and craft galleries, restaurants and tea rooms. > Richmond is a perfect place to learn about Tasmania’s rich heritage and is home to Australia’s oldest bridge, built by convict labour between 1823 and 1825, Australia’s oldest still-standing Catholic Church - St Johns - built in 1836, and Australia’s oldest gaol, built in 1825. > Also of interest is Old Hobart Town, a carefully constructed model of Hobart as it was in 1820. > Close by are the vineyards and wineries of the Coal River Valley, part of the Southern Tasmanian Wine Regions where you can taste award-winning cool-climate wines. > The region is also rich in produce including cheese and olives. Why not enjoy a long lunch at Frogmore Creek or Pooley Wines. Afterwards enjoy a row on the river or a relax on the riverbanks. > Overnight Richmond or return Hobart #discovertasmania fb.com/discovertasmania @tasmania WWW.DISCOVERTASMANIA.COM.AU Richmond - Port Arthur > On the way to Port Arthur stop at the Colonial and Convict Exhibition in Copping, with its extensive collection of interesting convict artifacts. -

Plant Charts for Native to the West Booklet
26 Pohutukawa • Oi exposed coastal ecosystem KEY ♥ Nurse plant ■ Main component ✤ rare ✖ toxic to toddlers coastal sites For restoration, in this habitat: ••• plant liberally •• plant generally • plant sparingly Recommended planting sites Back Boggy Escarp- Sharp Steep Valley Broad Gentle Alluvial Dunes Area ment Ridge Slope Bottom Ridge Slope Flat/Tce Medium trees Beilschmiedia tarairi taraire ✤ ■ •• Corynocarpus laevigatus karaka ✖■ •••• Kunzea ericoides kanuka ♥■ •• ••• ••• ••• ••• ••• ••• Metrosideros excelsa pohutukawa ♥■ ••••• • •• •• Small trees, large shrubs Coprosma lucida shining karamu ♥ ■ •• ••• ••• •• •• Coprosma macrocarpa coastal karamu ♥ ■ •• •• •• •••• Coprosma robusta karamu ♥ ■ •••••• Cordyline australis ti kouka, cabbage tree ♥ ■ • •• •• • •• •••• Dodonaea viscosa akeake ■ •••• Entelea arborescens whau ♥ ■ ••••• Geniostoma rupestre hangehange ♥■ •• • •• •• •• •• •• Leptospermum scoparium manuka ♥■ •• •• • ••• ••• ••• ••• ••• ••• Leucopogon fasciculatus mingimingi • •• ••• ••• • •• •• • Macropiper excelsum kawakawa ♥■ •••• •••• ••• Melicope ternata wharangi ■ •••••• Melicytus ramiflorus mahoe • ••• •• • •• ••• Myoporum laetum ngaio ✖ ■ •••••• Olearia furfuracea akepiro • ••• ••• •• •• Pittosporum crassifolium karo ■ •• •••• ••• Pittosporum ellipticum •• •• Pseudopanax lessonii houpara ■ ecosystem one •••••• Rhopalostylis sapida nikau ■ • •• • •• Sophora fulvida west coast kowhai ✖■ •• •• Shrubs and flax-like plants Coprosma crassifolia stiff-stemmed coprosma ♥■ •• ••••• Coprosma repens taupata ♥ ■ •• •••• •• -

Tasman Peninsula
7 A OJ? TASMAN PENINSULA M.R. Banks, E.A. Calholln, RJ. Ford and E. Williams University of Tasmania (MRB and the laie R.J. Ford). b!ewcastle fo rmerly University of Tasmama (EAC) and (ie,a/Ogle,Cl; Survey of Tasmania (E'W) (wjth two text-figures lUld one plate) On Tasman Peninsula, southeastern Tasmania, almost hOrizontal Permian marine and Triassic non-marine lOcks were inllUded by Jurassic dolerite, faulted and overiain by basalt Marine processes operating on the Jurassic and older rocks have prcl(iU!ced with many erosional features widely noted for their grandeur a self-renewing economic asset. Key Words: Tasman Peninsula, Tasmania, Permian, dolerite, erosional coastline, submarine topography. From SMITH, S.J. (Ed.), 1989: IS lllSTORY ENOUGH ? PA ST, PRESENT AND FUTURE USE OF THE RESOURCES OF TA SMAN PENINSULA Royal Society of Tasmania, Hobart: 7-23. INTRODUCTION Coal was discovered ncar Plunkett Point by surveyors Woodward and Hughes in 1833 (GO 33/ Tasman Peninsula is known for its spectacular coastal 16/264·5; TSA) and the seam visited by Captain scenery - cliffs and the great dolerite columns O'Hara Booth on May 23, 1833 (Heard 1981, p.158). which form cliffs in places, These columns were Dr John Lhotsky reported to Sir John Franklin on the first geological features noted on the peninsula. this coal and the coal mining methods in 1837 (CSO Matthew Flinders, who saw the columns in 1798, 5/72/1584; TSA). His thorough report was supported reported (1801, pp.2--3) that the columns at Cape by a coloured map (CSO 5/11/147; TSA) showing Pillar, Tasman Island and Cape "Basaltcs" (Raoul) some outcrops of different rock This map, were "not strictlybasaltes", that they were although not the Australian not the same in form as those Causeway Dictionary of (Vol. -

The Fern Family Blechnaceae: Old and New
ANDRÉ LUÍS DE GASPER THE FERN FAMILY BLECHNACEAE: OLD AND NEW GENERA RE-EVALUATED, USING MOLECULAR DATA Tese apresentada ao Programa de Pós-Graduação em Biologia Vegetal do Departamento de Botânica do Instituto de Ciências Biológicas da Universidade Federal de Minas Gerais, como requisito parcial à obtenção do título de Doutor em Biologia Vegetal. Área de Concentração Taxonomia vegetal BELO HORIZONTE – MG 2016 ANDRÉ LUÍS DE GASPER THE FERN FAMILY BLECHNACEAE: OLD AND NEW GENERA RE-EVALUATED, USING MOLECULAR DATA Tese apresentada ao Programa de Pós-Graduação em Biologia Vegetal do Departamento de Botânica do Instituto de Ciências Biológicas da Universidade Federal de Minas Gerais, como requisito parcial à obtenção do título de Doutor em Biologia Vegetal. Área de Concentração Taxonomia Vegetal Orientador: Prof. Dr. Alexandre Salino Universidade Federal de Minas Gerais Coorientador: Prof. Dr. Vinícius Antonio de Oliveira Dittrich Universidade Federal de Juiz de Fora BELO HORIZONTE – MG 2016 Gasper, André Luís. 043 Thefern family blechnaceae : old and new genera re- evaluated, using molecular data [manuscrito] / André Luís Gasper. – 2016. 160 f. : il. ; 29,5 cm. Orientador: Alexandre Salino. Co-orientador: Vinícius Antonio de Oliveira Dittrich. Tese (doutorado) – Universidade Federal de Minas Gerais, Departamento de Botânica. 1. Filogenia - Teses. 2. Samambaia – Teses. 3. RbcL. 4. Rps4. 5. Trnl. 5. TrnF. 6. Biologia vegetal - Teses. I. Salino, Alexandre. II. Dittrich, Vinícius Antônio de Oliveira. III. Universidade Federal de Minas Gerais. Departamento de Botânica. IV. Título. À Sabrina, meus pais e a vida, que não se contém! À Lucia Sevegnani, que não pode ver esta obra concluída, mas que sempre foi motivo de inspiração. -

Shearing the Waratah: “Cornish” Tin Recovery on the Arthur River System, Tasmania, 1878–1903
Journal of Australasian Mining History, Vol. 15, October 2017 Shearing the Waratah: “Cornish” tin recovery on the Arthur River system, Tasmania, 1878–1903 By NIC HAYGARTH University of Tasmania ourist guff about Tasmania’s Arthur River insists that it is ‘wild’, having never been ‘farmed, logged, mined or dammed’.1 The Arthur’s forested lower reaches, T which are cruised by tourist vessels give the appearance of being in a natural state, with abundant native bird and animal life. Stands of miraculously preserved ancient rainforest near its middle reaches host guided bushwalking experiences. This is the so-called Tarkine Wilderness. Map 1: Mount Bischoff and the Waratah River, showing the position of rival companies Source: Courtesy of Department of Primary Industries, Parks, Water & Environment, Tasmania. Other parts of the Arthur River system are more like an industrial wilderness. The tiny stream begins its passage to the Southern Ocean by trickling out of the Magnet silver-lead mine’s no. 1 dam, then receives Magnet Creek, half of which spews, stinking of sulphur, from the Magnet mine’s South Adit, plus the alarmingly yellow Tinstone 81 Nic Haygarth Creek, which drains the Bischoff Extended tin workings. Further downstream, the Arthur receives the Waratah River, which in 1910 was declared a ‘sludge channel’, effectively abrogating the Mount Bischoff Tin Mining Company (Mount Bischoff Co.) of responsibility for its various water-borne discharges.2 Before remediation was undertaken by Mineral Resources Tasmania, Mount Bischoff drainage -

Pteridophyte Fungal Associations: Current Knowledge and Future Perspectives
This is a repository copy of Pteridophyte fungal associations: Current knowledge and future perspectives. White Rose Research Online URL for this paper: http://eprints.whiterose.ac.uk/109975/ Version: Accepted Version Article: Pressel, S, Bidartondo, MI, Field, KJ orcid.org/0000-0002-5196-2360 et al. (2 more authors) (2016) Pteridophyte fungal associations: Current knowledge and future perspectives. Journal of Systematics and Evolution, 54 (6). pp. 666-678. ISSN 1674-4918 https://doi.org/10.1111/jse.12227 © 2016 Institute of Botany, Chinese Academy of Sciences. This is the peer reviewed version of the following article: Pressel, S., Bidartondo, M. I., Field, K. J., Rimington, W. R. and Duckett, J. G. (2016), Pteridophyte fungal associations: Current knowledge and future perspectives. Jnl of Sytematics Evolution, 54: 666–678., which has been published in final form at https://doi.org/10.1111/jse.12227. This article may be used for non-commercial purposes in accordance with Wiley Terms and Conditions for Self-Archiving. Reuse Unless indicated otherwise, fulltext items are protected by copyright with all rights reserved. The copyright exception in section 29 of the Copyright, Designs and Patents Act 1988 allows the making of a single copy solely for the purpose of non-commercial research or private study within the limits of fair dealing. The publisher or other rights-holder may allow further reproduction and re-use of this version - refer to the White Rose Research Online record for this item. Where records identify the publisher as the copyright holder, users can verify any specific terms of use on the publisher’s website. -

Pteridologist 2009
PTERIDOLOGIST 2009 Contents: Volume 5 Part 2, 2009 The First Pteridologist Alec Greening 66 Back from the dead in Corrie Fee Heather McHaffie 67 Fern fads, fashions and other factors Alec Greening 68 A Stumpery on Vashon Island near Seattle Pat Reihl 71 Strange Revisions to The Junior Oxford English Dictionary Alistair Urquhart 73 Mauchline Fern Ware Jennifer Ide 74 More Ferns In Unusual Places Bryan Smith 78 The Ptéridophytes of Réunion Edmond Grangaud 79 Croziers - a photographic study. Linda Greening 84 A fern by any other name John Edgington 85 Tree-Fern Newsletter No. 15 Edited by Alastair C. Wardlaw 88 Editorial: TFNL then and now Alastair C. Wardlaw 88 Courtyard Haven for Tree Ferns Alastair C. Wardlaw 88 Bulbils on Tree Ferns: II Martin Rickard 90 Gough-Island Tree Fern Comes to Scotland Jamie Taggart 92 Growing ferns in a challenging climate Tim Pyner 95 Maraudering caterpillars. Yvonne Golding 104 New fern introductions from Fibrex Nurseries Angela Tandy 105 Ferns which live with ants! Yvonne Golding 108 The British Fern Gazette 1909 – 2009 Martin Rickard 110 A Siberian Summer Chris Page 111 Monitoring photographs of Woodsia ilvenis Heather McHaffie 115 Notes on Altaian ferns Irina Gureyeva 116 Ferns from the Galapagos Islands. Graham Ackers 118 Did you know? (Extracts from the first Pteridologist) Jimmy Dyce 121 The First Russian Pteridological Conference Chris Page 122 Tectaria Mystery Solved Pat Acock 124 Chatsworth - a surprising fern link with the past Bruce Brown 125 Fern Postage Stamps from the Faroe Islands Graham Ackers 127 Carrying out trials in your garden Yvonne Golding 128 A national collection of Asplenium scolopendrium Tim Brock 130 Asplenium scolopendrium ‘Drummondiae’ Tim Brock 132 Fern Recording – A Personal Scottish Experience Frank McGavigan 133 Book Notes Martin Rickard 136 Gay Horsetails Wim de Winter 137 Ferning in snow Martin Rickard 139 Fern Enthusiasts do the strangest things.