A Case of Inversion and Duplication Involving Constitutive Heterochromatin
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

N.C. Turtles Checklist
Checklist of Turtles Historically Encountered In Coastal North Carolina by John Hairr, Keith Rittmaster and Ben Wunderly North Carolina Maritime Museums Compiled June 1, 2016 Suborder Family Common Name Scientific Name Conservation Status Testudines Cheloniidae loggerhead Caretta caretta Threatened green turtle Chelonia mydas Threatened hawksbill Eretmochelys imbricata Endangered Kemp’s ridley Lepidochelys kempii Endangered Dermochelyidae leatherback Dermochelys coriacea Endangered Chelydridae common snapping turtle Chelydra serpentina Emydidae eastern painted turtle Chrysemys picta spotted turtle Clemmys guttata eastern chicken turtle Deirochelys reticularia diamondback terrapin Malaclemys terrapin Special concern river cooter Pseudemys concinna redbelly turtle Pseudemys rubriventris eastern box turtle Terrapene carolina yellowbelly slider Trachemys scripta Kinosternidae striped mud turtle Kinosternon baurii eastern mud turtle Kinosternon subrubrum common musk turtle Sternotherus odoratus Trionychidae spiny softshell Apalone spinifera Special concern NOTE: This checklist was compiled and updated from several sources, both in the scientific and popular literature. For scientific names, we have relied on: Turtle Taxonomy Working Group [van Dijk, P.P., Iverson, J.B., Rhodin, A.G.J., Shaffer, H.B., and Bour, R.]. 2014. Turtles of the world, 7th edition: annotated checklist of taxonomy, synonymy, distribution with maps, and conservation status. In: Rhodin, A.G.J., Pritchard, P.C.H., van Dijk, P.P., Saumure, R.A., Buhlmann, K.A., Iverson, J.B., and Mittermeier, R.A. (Eds.). Conservation Biology of Freshwater Turtles and Tortoises: A Compilation Project of the IUCN/SSC Tortoise and Freshwater Turtle Specialist Group. Chelonian Research Monographs 5(7):000.329–479, doi:10.3854/crm.5.000.checklist.v7.2014; The IUCN Red List of Threatened Species. -

Turtles, All Marine Turtles, Have Been Documented Within the State’S Borders
Turtle Only four species of turtles, all marine turtles, have been documented within the state’s borders. Terrestrial and freshwater aquatic species of turtles do not occur in Alaska. Marine turtles are occasional visitors to Alaska’s Gulf Coast waters and are considered a natural part of the state’s marine ecosystem. Between 1960 and 2007 there were 19 reports of leatherback sea turtles (Dermochelys coriacea), the world’s largest turtle. There have been 15 reports of Green sea turtles (Chelonia mydas). The other two are extremely rare, there have been three reports of Olive ridley sea turtles (Lepidochelys olivacea) and two reports of loggerhead sea turtles (Caretta caretta). Currently, all four species are listed as threatened or endangered under the U.S. Endangered Species Act. Prior to 1993, Alaska marine turtle sightings were mostly of live leatherback sea turtles; since then most observations have been of green sea turtle carcasses. At present, it is not possible to determine if this change is related to changes in oceanographic conditions, perhaps as the result of global warming, or to changes in the overall population size and distribution of these species. General description: Marine turtles are large, tropical/subtropical, thoroughly aquatic reptiles whose forelimbs or flippers are specially modified for swimming and are considerably larger than their hind limbs. Movements on land are awkward. Except for occasional basking by both sexes and egg-laying by females, turtles rarely come ashore. Turtles are among the longest-lived vertebrates. Although their age is often exaggerated, they probably live 50 to 100 years. Of the five recognized species of marine turtles, four (including the green sea turtle) belong to the family Cheloniidae. -

Harvested Testudines
Testudines HARVESTED – Is the species collected by humans? Species Common Name Harvested Cheloniidae sea turtles Caretta c. caretta Atlantic loggerhead Unk Chelonia m. mydas Atlantic green turtle YF Economically Chelonia mydas is the most important reptile in the world. Its flesh and its eggs serve as an important source of protein in many third-world nations where protein is scarce (Ernst et al. 1994) Eretmochelys i. imbricata Atlantic hawksbill YF hawksbill flesh and eggs are eaten in many parts of its range (Ernst et al. 1994); YC throughout its range it is hunted for the plates of its shells (Ernst et al. 1994) Lepidochelys kempii Kemp’s ridley or Atlantic ridley YF egg poaching caused the initial decline [in population size] (Delikat 1981; Hall et al. 1983; Lutz and Lutcavage 1989; Ross et al. 1989; Thompson 1989; National Research Council 1990; Caillouet et al. 1991) Dermochelyidae leatherback sea turtles Dermochelys c. coriacea Atlantic leatherback YF gathering the eggs for both commerce and personal consumption (Ernst et al. 1994) Chelydridae snapping turtles Chelydra s. serpentina eastern snapping turtle YF the flesh of the snapping turtle is tasty and eaten throughout the range…fresh eggs are edible if fried (Ernst et al. 1994) Emydidae pond turtles Chrysemys p. picta eastern painted turtle Unk Chrysemys p. marginata midland painted turtle Unk Clemmys guttata spotted turtle YP (Lovich and Jaworski 1988; Lovich 1989; J. Harding, pers. comm.) Clemmys insculpta wood turtle YP (Ernst et al. 1994) Clemmys muhlenbergii bog turtle YP (D.E. Collins 1990); YF eaten by people (Ernst et al. 1994) Deirochelys r. -

Reproductive Physiology of Nesting Leatherback Turtles (Dermochelys Coriacea) at Las Baulas National Park, Costa Rica
Chelolliall COllserwuioll (lnd Biology, 1996,2(2):230- 236 © 1996 by Chelonian Research Foundation Reproductive Physiology of Nesting Leatherback Turtles (Dermochelys coriacea) at Las Baulas National Park, Costa Rica i 2 3 4 DAVID C. ROSTAL , FRANK V. PALADIN0 , RHONDA M. PATTERSON , AND JAMES R. SPOTILA I Department of Biology, Georgia Southern Uni versity, Statesboro, Georgia 30460 USA [Fax: 912-681-0845; E-mail: [email protected]}; 2Department of Biology, Indiana-Purdue University, Fort Wayne, Indiana 46805 USA ; 3Department of Biology, Texas A&M University, College Station, Texas 77843 USA ; 4Department o{Bioscience and Biotechnology, Drexel University, Philadelphia, Pennsylvania 19104 USA ABSTRACT. - The reproductive physiology of nesting leatherback turtles (Dermochelys coriacea) was studied at Playa Grande, Costa Rica, from 1992 to 1994 during November, December, and January. Ultrasonography indicated that 82 % of nesting females had mature preovulatory ovaries in November, 40 % in December, and 23 % in January. Mean follicular diameter was 3.33 ±0.02 cm and did not vary significantly through the nesting season. Plasma testosterone and estradiol levels measured by radioimmunoassay correlated strongly with reproductive condition. No correlation, however, was observed between reproductive condition and plasma progesterone levels. Testoster one declined from 2245 ± 280 pglml at the beginning of the nesting cycle to 318 ± 89 pglml at the end of the nesting cycle. Estradiol declined in a similar manner. Plasma calcium levels were constant throughout the nesting cycle. Vitellogenesis appeared complete prior to the arrival of the female at the nesting beach. Dermochelys coriacea is a seasonal nester displaying unique variations (e.g., 9 to 10 day internesting interval, yolkless eggs) on the basic chelonian pattern. -

Ecol 483/583 – Herpetology Lab 11: Reptile Diversity 3: Testudines and Crocodylia Spring 2010
Ecol 483/583 – Herpetology Lab 11: Reptile Diversity 3: Testudines and Crocodylia Spring 2010 P.J. Bergmann & S. Foldi Lab objectives The objectives of today’s lab are to: 1. Familiarize yourselves with extant diversity of the Testudines and Crocodylia. 2. Learn to identify species of Testundines that live in Arizona. Today's lab is the final lab on "reptile" diversity, and will introduce you to the Testudines, or turtles and tortoises, Crocodylia. Although there are no crocodylians in Arizona and the Testudine diversity is lower than that of the lizards or snakes, there is still a fair amount of material to learn, so use your time wisely. Tips for learning the material At this point in the course, your skills for learning herp diversity should be well honed, so continue with the strategies you have already learned during the semester. Learn how to differentiate the three major clades of Crocodylians, and the more numerous Testudine clades. There is another keying exercise in this lab, focusing on the Testudines. 1 Ecol 483/583 – Lab 11: Testudines & Crocs 2010 Exercise 1: Testudines (Modified from Bonine & Foldi 2008; Bonine, Dee & Hall 2006; Edwards 2002; Prival 2000) General information Turtles are probably the most instantly recognizable groups of all reptiles because of their shell and the ability to withdraw their heads and limbs into this protective structure. Turtles are a monophyletic group comprising the order Testudines also called Chelonia . Testudines is the term used to denote all members of the order (extant and extinct) whereas Chelonia is often used to denote extant turtles. -

Comparative Bone Histology of the Turtle Shell (Carapace and Plastron)
Comparative bone histology of the turtle shell (carapace and plastron): implications for turtle systematics, functional morphology and turtle origins Dissertation zur Erlangung des Doktorgrades (Dr. rer. nat.) der Mathematisch-Naturwissenschaftlichen Fakultät der Rheinischen Friedrich-Wilhelms-Universität zu Bonn Vorgelegt von Dipl. Geol. Torsten Michael Scheyer aus Mannheim-Neckarau Bonn, 2007 Angefertigt mit Genehmigung der Mathematisch-Naturwissenschaftlichen Fakultät der Rheinischen Friedrich-Wilhelms-Universität Bonn 1 Referent: PD Dr. P. Martin Sander 2 Referent: Prof. Dr. Thomas Martin Tag der Promotion: 14. August 2007 Diese Dissertation ist 2007 auf dem Hochschulschriftenserver der ULB Bonn http://hss.ulb.uni-bonn.de/diss_online elektronisch publiziert. Rheinische Friedrich-Wilhelms-Universität Bonn, Januar 2007 Institut für Paläontologie Nussallee 8 53115 Bonn Dipl.-Geol. Torsten M. Scheyer Erklärung Hiermit erkläre ich an Eides statt, dass ich für meine Promotion keine anderen als die angegebenen Hilfsmittel benutzt habe, und dass die inhaltlich und wörtlich aus anderen Werken entnommenen Stellen und Zitate als solche gekennzeichnet sind. Torsten Scheyer Zusammenfassung—Die Knochenhistologie von Schildkrötenpanzern liefert wertvolle Ergebnisse zur Osteoderm- und Panzergenese, zur Rekonstruktion von fossilen Weichgeweben, zu phylogenetischen Hypothesen und zu funktionellen Aspekten des Schildkrötenpanzers, wobei Carapax und das Plastron generell ähnliche Ergebnisse zeigen. Neben intrinsischen, physiologischen Faktoren wird die -

Herptiles of Rhode Island, Checklist for Use in Bioblitz Bufo Americanus
Herptiles of Rhode Island, Checklist for use in BioBlitz From Vertebrates of Rhode Island, August, Enser, Gould, 2001, Pub. by RINHS Initials BUFONIDAE – Toads Bufo americanus (American Toad) B. fowleri (Fowler's Toad) HYLIDAE - Treefrogs Hyla versicolor (Gray Treefrog) Pseudacris crucifer crucifer (Northern Spring Peeper) RANIDAE - True Frogs Rana catesbeiana (Bullfrog) R. clamitans melanota (Green Frog) R. palustris (Pickerel Frog) R. pipiens (Northern Leopard Frog) R. sylvatica (Wood Frog) SCAPHIOPODIDAE - Spadefoots Scaphiopus holbrookii (Eastern Spadefoot) AMBYSTOMATIDAE - Mole Salamanders Ambystoma maculatum (Spotted Salamander) A. opacum (Marbled Salamander) PLETHODONTIDAE - Lungless Salamanders Desmognathus fuscus (Northern Dusky Salamander) Eurycea bislineata (Northern Two-lined Salamander) Gyrinophilus p. porphyriticus (Northern Spring Salr) Hemidactylium scutatum (Four-toed Salamander) Plethodon cinereus (Northern Red-backed Salamander) SALAMANDRIDAE - Newts Notophthalmus v. viridescens (Red-spotted Newt) COLUBRIDAE - Colubrid Snakes Carphophis amoenus amoenus (Eastern Worm Snake) Coluber constrictor constrictor (Northern Black Racer) Diadophis punctatus edwardsii (Northern Ringneck Snake) Elaphe obsoleta obsoleta (Black Rat Snake) Heterodon platirhinos (Eastern Hognose Snake) Lampropeltis triangulum triangulum (Eastern Milk Snake) Liochlorophis vernalis (Smooth Green Snake) Nerodia sipedon sipedon (Northern Water Snake) Storeria dekayi dekayi (Northern Brown Snake) S. o. occipitomaculata (Northern Redbelly Snake) Thamnophis -

(Chelonioidea: Cheloniidae) from the Maastrichtian of the Harrana Fauna–Jordan
Kaddumi, Gigantatypus salahi n.gen., n.sp., from Harrana www.PalArch.nl, vertebrate palaeontology, 3, 1, (2006) A new genus and species of gigantic marine turtles (Chelonioidea: Cheloniidae) from the Maastrichtian of the Harrana Fauna–Jordan H.F. Kaddumi Eternal River Museum of Natural History Amman–Jordan, P.O. Box 11395 [email protected] ISSN 1567–2158 7 figures Abstract Marine turtle fossils are extremely rare in the Muwaqqar Chalk Marl Formation of the Harrana Fauna in comparison to the relatively rich variety of other vertebrate fossils collected from this locality. This paper reports and describes the remains of an extinct marine turtle (Chelonioidea) which will be tentatively assigned to a new genus and species of marine turtles (Cheloniidae Bonaparte, 1835) Gigantatypus salahi n.gen., n.sp.. The new genus represented by a single well–preserved right humerus, reached remarkably large proportions equivalent to that of Archelon Wieland, 1896 and represents the first to be found from this deposit and from the Middle East. The specimen, which exhibits unique combinations of features is characterized by the following morphological features not found in other members of the Cheloniidae: massive species reaching over 12 feet in length; a more prominently enlarged lateral process that is situated more closely to the head; a ventrally situated capitellum; a highly laterally expanded distal margin. The presence of these features may warrant the placement of this new species in a new genus. The specimen also retains some morphological features found in members of advanced protostegids indicating close affinities with the family. Several bite marks on the ventral surface of the fossilized humerus indicate shark–scavenging activities of possibly Squalicorax spp. -

Taxonomy and Phylogeny of the Higher Categories of Cryptodiran Turtles Based on a Cladistic Analysis of Chromosomal Data
Copein, 1983(4), pp. 918-932 Taxonomy and Phylogeny of the Higher Categories of Cryptodiran Turtles Based on a Cladistic Analysis of Chromosomal Data John W. Bickham and John L. Carr Karyological data are available for 55% of all cryptodiran turtle species including members of all but one family. Cladistic analysis of these data, as well as con sideration of other taxonomic studies, lead us to propose a formal classification and phylogeny not greatly different from that suggested by other workers. We recognize 11 families and three superfamilies. The platysternid and staurotypid turtles are recognized at the familial level. Patterns and models of karyotypic evolution in turtles are reviewed and discussed. OVER the past 10 years knowledge of turtle and the relationship between the shell and pel karyology has grown to such an extent vic girdle. In the cryptodires ("hidden-necked" that the order Testudines is one of the better turtles), the neck is withdrawn into the body in known groups of lower vertebrates (Bickham, a vertical plane and the pelvis is not fused to 1983). Nondifferentially stained karyotypes are either the plastron or carapace, whereas in the known for 55% of cryptodiran turtle species pleurodires ("side-necked" turtles) the pelvic and banded karyotypes for approximately 25% girdle is fused to both the plastron and carapace (Bickham, 1981). From this body of knowledge, and the neck is folded back against the body in as well as a consideration of the morphological a horizontal plane. Cope's suborder Athecae variation in the order, we herein present a gen includes only the Dermochelyidae and is no eral review of the cryptodiran karyological lit longer recognized. -

Leatherback Sea Turtle
VOCABULARY Carapace: the dorsal (upper) part of the shell, covered by LEATHERBACK SEA TURTLE bony external plates called scutes (Dermochelys coriacea) Critical habitat: Locations mandated for the conservation Class: Reptilia of endangered species flippers lack Order: Testudines claws and Hatchling: a baby turtle scales Family: Dermochelyidae Pelagic: Living in open Genius: Dermochelys ` ocean as opposed to Species: coriacea coastal waters near land Plastron: the ventral (underside) part of the shell,also covered in Leatherbacks are the only sea scutes turtles that lack hard, bony shells. seven longitudinal ridges long flippers help travel long distances PHYSICAL DESCRIPTION The largest sea turtles in the world, leatherbacks can grow up In a single nesting season, females lay about 100 eggs at as many as to 6.5 feet long and weigh close to 2,000 pounds. Adults nine different locations. are black with pink and white speckled plastrons, with simi- lar coloring on the tops of their heads. Their carapaces are covered by thick, leathery tissue and has seven longitudinal ridges. Flippers lack claws and scales. DIET These foragers migrate long distances to find food. Their mouths have pointed, tooth-like cusps with sharp- edged jaws perfectly designed for eating soft prey. They Hatchlings have distinct white mark- feed mostly on jellyfish, but also eat octopus and tunicates. ings along their carapace ridges. LIFE HISTORY They mate in tropical coastal waters near nesting beaches, where females lay their eggs in the sand. Hatchlings emerge 60-65 days later and make their way to the ocean. GEOGRAPHICAL DISTRIBUTION Leatherbacks are the TO REPORT A STRANDED MARINE world’s most wide-ranging sea turtles. -
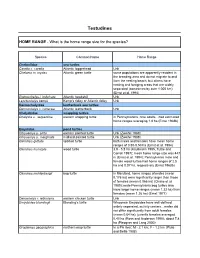
Home Range Testudines
Testudines HOME RANGE - What is the home range size for the species? Species Common Name Home Range Cheloniidae sea turtles Caretta c. caretta Atlantic loggerhead Unk Chelonia m. mydas Atlantic green turtle some populations are apparently resident in the breeding area and do not migrate to and from the nesting beach, but others have nesting and foraging areas that are widely separated (sometimes by over 1,000 km) (Ernst et al. 1994) Eretmochelys i. imbricata Atlantic hawksbill Unk Lepidochelys kempii Kemp’s ridley or Atlantic ridley Unk Dermochelyidae leatherback sea turtles Dermochelys c. coriacea Atlantic leatherback Unk Chelydridae snapping turtles Chelydra s. serpentina eastern snapping turtle in Pennsylvanina, nine adults…had estimated home ranges averaging 1.8 ha (Ernst 1968b) Emydidae pond turtles Chrysemys p. picta eastern painted turtle Unk (Zweifel 1989) Chrysemys p. marginata midland painted turtle Unk (Zweifel 1989) Clemmys guttata spotted turtle both males and females have mean home ranges of 0.50-0.53 ha (Ernst et al. 1994) Clemmys insculpta wood turtle 3.9 - 5.8 ha (Kaufmann 1995, Tuttle and Carroll 1997); mean home range size was 447 m (Ernst et al. 1994); Pennsylvania male and female wood turtles had home ranges of 2.5 ha and 0.07 ha, respectively (Ernst 1968b) Clemmys muhlenbergii bog turtle in Maryland, home ranges of males (mean 0.176 ha) were significantly larger than those of females (mean 0.066 ha) (Chase et al. 1989); male Pennsylvania bog turtles also have larger home ranges (mean 1.33 ha) than females (mean 1.26 ha) (Ernst 1977) Deirochelys r. -

The Characterization of Tortoise Shell and Its Imitations
THE CHARACTERIZATION OF TORTOISE SHELL AND ITS IMITATIONS Thomas Hainschwang and Laurence Leggio Tortoise shell has long been used as an ornamental gem material for art objects, jewelry, and personal items such as combs and eyeglass frames. Though humans have used tortoise shell for thousands of years, the material reached the height of its popularity during the 18th, 19th, and early 20th centuries. The advent of plastic imitations, as well as the passage of laws protecting sea turtles beginning in the 1970s, have led to a drastic reduction in the amount of tortoise shell in the market. Nevertheless, because older material can still be traded, especially in antique pieces, and because numerous imitations exist, proper identification is still important. This study summarizes the gemological properties of tortoise shell and its imitations. In addition to standard gemological data, the results of several spectroscopic techniques are presented; transmission and specular reflectance infrared spectroscopy were found to be of particular value. he term “tortoise shell” generally refers to the patches, though a homogeneous “cream”-colored carapacial (dorsal shell) and plastron (belly) variety called blond tortoise shell also exists. The Tplates of the hawksbill sea turtle (Eretmo- popularity of tortoise shell during the 18th through chelys imbricata). The use of tortoise shell dates at early 20th centuries caused these animals to be hunt- least to pre-dynastic Egypt (3500–3100 BC), from ed almost to extinction, leading to a near-worldwide which period dishes, combs, bracelets, and the like ban on collection in the 1970s, as well as a ban on are known (“Animal products...,” 2005).