THE STRUCTURED STORAGE of ONCOLOGICAL CHEMOTERAPEUTIC REGIMENS Contribution to Standardization of Therapeutic Procedures in Current Oncology
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

PI3K Inhibitors in Cancer: Clinical Implications and Adverse Effects
International Journal of Molecular Sciences Review PI3K Inhibitors in Cancer: Clinical Implications and Adverse Effects Rosalin Mishra , Hima Patel, Samar Alanazi , Mary Kate Kilroy and Joan T. Garrett * Department of Pharmaceutical Sciences, College of Pharmacy, University of Cincinnati, Cincinnati, OH 45267-0514, USA; [email protected] (R.M.); [email protected] (H.P.); [email protected] (S.A.); [email protected] (M.K.K.) * Correspondence: [email protected]; Tel.: +1-513-558-0741; Fax: +1-513-558-4372 Abstract: The phospatidylinositol-3 kinase (PI3K) pathway is a crucial intracellular signaling pathway which is mutated or amplified in a wide variety of cancers including breast, gastric, ovarian, colorectal, prostate, glioblastoma and endometrial cancers. PI3K signaling plays an important role in cancer cell survival, angiogenesis and metastasis, making it a promising therapeutic target. There are several ongoing and completed clinical trials involving PI3K inhibitors (pan, isoform-specific and dual PI3K/mTOR) with the goal to find efficient PI3K inhibitors that could overcome resistance to current therapies. This review focuses on the current landscape of various PI3K inhibitors either as monotherapy or in combination therapies and the treatment outcomes involved in various phases of clinical trials in different cancer types. There is a discussion of the drug-related toxicities, challenges associated with these PI3K inhibitors and the adverse events leading to treatment failure. In addition, novel PI3K drugs that have potential to be translated in the clinic are highlighted. Keywords: cancer; PIK3CA; resistance; PI3K inhibitors Citation: Mishra, R.; Patel, H.; Alanazi, S.; Kilroy, M.K.; Garrett, J.T. -

A Phase II Trial of the Src-Kinase Inhibitor AZD0530 in Patients with Advanced Castration-Resistant Prostate Cancer: a California Cancer Consortium Study Primo N
Clinical report 179 A phase II trial of the Src-kinase inhibitor AZD0530 in patients with advanced castration-resistant prostate cancer: a California Cancer Consortium study Primo N. Lara Jra,b, Jeff Longmatec, Christopher P. Evansa, David I. Quinnd, Przemyslaw Twardowskic, Gurkamal Chattae, Edwin Posadasf, Walter Stadlerf and David R. Gandaraa Prostate cancer cells undergo neuroendocrine AZD0530, a potent oral Src kinase inhibitor, is feasible differentiation during androgen deprivation and secrete and tolerable in this pretreated patient population neuropeptides, hence activating androgen receptor- but possessed little clinical efficacy as monotherapy. regulated genes. Src-family protein kinases are involved in Strong preclinical evidence warrants further neuropeptide-induced prostate cancer growth and investigation of AZD0530 in earlier-stage prostate migration. A phase II trial of AZD0530, an oral Src-family cancer or as combination therapy. Anti-Cancer Drugs kinase inhibitor, in patients with advanced castration 20:179–184 c 2009 Wolters Kluwer Health | Lippincott resistant prostate cancer was conducted. The primary Williams & Wilkins. endpoint was prostate cancer-specific antigen (PSA) response rate, defined as a 30% or greater decrease. Anti-Cancer Drugs 2009, 20:179–184 A two-stage Simon design was used. Eligibility criteria Keywords: castration-resistant, prostate cancer, Src inhibitor included documentation of castration resistance (including antiandrogen withdrawal), adequate end-organ aUniversity of California Davis Cancer Center, Sacramento, bVeterans’ function, and performance status, and not more than one Administration of Northern California, Mather, cCity of Hope Comprehensive Cancer Center, Duarte, dUniversity of Southern California Norris Cancer Center, prior taxane-based chemotherapy regimen. AZD0530 was Los Angeles, California, eUniversity of Pittsburgh Cancer Center, Pittsburgh, given at 175 mg orally once daily continuously. -

Is Combined Chemotherapy with Cisplatin, Etoposide and Irinotecan the New Standard Treatment for Patients with Sensitive Relapsed Small Cell Lung Cancer?
Editorial Is combined chemotherapy with cisplatin, etoposide and irinotecan the new standard treatment for patients with sensitive relapsed small cell lung cancer? Alessandro Morabito Oncologia Medica Toraco-Polmonare, IRCCS Istituto Nazionale dei Tumori, Fondazione Pascale, Napoli, Italy Correspondence to: Alessandro Morabito, MD. Director of Thoracic Medical Oncology, National Cancer Institute, Via Mariano Semola, 80131 Naples, Italy. Email: [email protected]; [email protected]. Comment on: Goto K, Ohe Y, Shibata T, et al. Combined chemotherapy with cisplatin, etoposide, and irinotecan versus topotecan alone as second-line treatment for patients with sensitive relapsed small-cell lung cancer (JCOG0605): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2016;17:1147-57. Submitted Nov 20, 2016. Accepted for publication Dec 01, 2016. doi: 10.21037/tcr.2016.12.37 View this article at: http://dx.doi.org/10.21037/tcr.2016.12.37 Treatment of small cell lung cancer (SCLC) remains a best supportive care (BSC), combined chemotherapy with significant challenge for the oncologists. Attempts to cyclophosphamide, doxorubicin and vincristine (CAV), improve the results of first- and second-line treatment oral topotecan and amrubicin: topotecan improved OS have all failed so far and no real progress has been made and quality of life compared with BSC, while CAV and in last years, emphasizing the need for novel strategies amrubicin did not show any survival benefit compared with of treatment. Patients with relapsed SCLC are usually topotecan (6-9). Although the efficacy of topotecan was low, classified into different categories, according to the time with response rates from 7% to 24% and OS from 5.8 to elapsed from the end of previous treatment: sensitive, if 9.9 months, no regimen showed superiority over topotecan tumor progression is documented at least 3 months after that continues to be considered as the standard second-line the completion of initial treatment, or resistant if tumor chemotherapy for patients with relapsed SCLC. -

Taxotere (Docetaxel) Label
Patient Information Leaflet Detach and give to Patient Rev.May 2004 Patient Information Leaflet Questions and Answers About Taxotere® Injection Concentrate (generic name = docetaxel) (pronounced as TAX-O-TEER) What is Taxotere? Taxotere is a medication to treat breast cancer, non-small cell lung cancer and prostate cancer. It has severe side effects in some patients. This leaflet is designed to help you understand how to use Taxotere and avoid its side effects to the fullest extent possible. The more you understand your treatment, the better you will be able to participate in your care. If you have questions or concerns, be sure to ask your doctor or nurse. They are always your best source of information about your condition and treatment. What is the most important information about Taxotere? • Since this drug, like many other cancer drugs, affects your blood cells, your doctor will ask for routine blood tests. These will include regular checks of your white blood cell counts. People with low blood counts can develop life-threatening infections. The earliest sign of infection may be fever, so if you experience a fever, tell your doctor right away. • Occasionally, serious allergic reactions have occurred with this medicine. If you have any allergies, tell your doctor before receiving this medicine. • A small number of people who take Taxotere have severe fluid retention, which can be life- threatening. To help avoid this problem, you must take another medication such as dexamethasone (DECKS-A-METH-A-SONE) prior to each Taxotere treatment. You must follow the schedule and take the exact dose of dexamethasone prescribed (see schedule at end of brochure). -

Advances in Cancer Research Hariharan U*
Editorial iMedPub Journals Archives in Cancer Research 2021 www.imedpub.com Vol.9 ISSN No.S2:e003 Advances in Cancer Research Hariharan U* Anesthesiology & Intensive Care, Dr Ram Manohar Lohia Hospital & ABVIMS-PGIMER, GGSIP University, New Delhi, India *Corresponding author: Uma Hariharan, Anesthesiology & Intensive Care, Dr Ram Manohar Lohia Hospital & PGIMER, New Delhi, India Tel: +91- 9811271093, E-mail: [email protected] Citation: Hariharan U, (2021) Advances in Cancer research. Arch Cancer Res.Vol.9 No.S2: e003. Copyright: © 2021 Hariharan U. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Introduction therapy for cancer in that they are introduced into the blood stream and are therefore in principle able to address cancer at Chemotherapy (often abbreviated to chemo and sometimes any anatomic location in the body. Systemic therapy is often CTX or CTx) is a type of cancer treatment that uses one or more used in conjunction with other modalities that constitute local anti-cancer drugs (chemotherapeutic agents) as part of a therapy (i.e. treatments whose efficacy is confined to the standardized chemotherapy regimen. Chemotherapy may be anatomic area where they are applied) for cancer such as given with a curative intent (which almost always involves radiation therapy, surgery or hyperthermia therapy. combinations of drugs), or it may aim to prolong life or to Traditional chemotherapeutic agents are cytotoxic by means reduce symptoms (palliative chemotherapy). Chemotherapy is of interfering with cell division (mitosis) but cancer cells vary one of the major categories of the medical discipline specifically widely in their susceptibility to these agents. -

DEXAMETHASONE – RITUXIMAB (DRC) Regimen • Lymphoma
Chemotherapy Protocol LYMPHOMA CYCLOPHOSPHAMIDE - DEXAMETHASONE – RITUXIMAB (DRC) Regimen • Lymphoma – DRC – Cyclophosphamide – Dexamethasone - Rituximab Indication • Lymphophlasmacytic lymphoma / Waldenstrom’s macroglobinaemia Toxicity Drug Adverse Effect Cyclophosphamide Dysuria, haemorrhagic cystitis (rare), taste disturbances Weight gain, gastrointestinal disturbances, hyperglycaemia, CNS Dexamethasone disturbances, Cushingoid changes, glucose intolerance. Severe cytokine release syndrome, increased incidence of Rituxumab infective complications, progressive multifocal leukoencephalopathy The adverse effects listed are not exhaustive. Please refer to the relevant Summary of Product Characteristics for full details. Monitoring Drugs • FBC, LFTs and U&Es prior to day one of treatment • Check hepatitis B status before starting treatment with rituximab • Regular monitoring of blood glucose is considered good practice. Dose Modifications The dose modifications listed are for haematological, liver and renal function and drug specific toxicities only. Dose adjustments may be necessary for other toxicities as well. In principle all dose reductions due to adverse drug reactions should not be re-escalated in subsequent cycles without consultant approval. It is also a general rule for chemotherapy that if a third dose reduction is necessary treatment should be stopped. Version 1 (September 2019) Page 1 of 10 Lymphoma – DRC – Cyclophosphamide – Dexamethasone - Rituximab Please discuss all dose reductions / delays with the relevant consultant before prescribing, if appropriate. The approach may be different depending on the clinical circumstances. Haematological Dose modifications for haematological toxicity in the table below are for general guidance only. Always refer to the responsible consultant as any dose reductions or delays will be dependent on clinical circumstances and treatment intent. Low counts can be a consequence of bone marrow infiltration as well as drug toxicity. -

Chemotherapy Regimens for Lymphoma
Helpline freephone 0808 808 5555 [email protected] www.lymphoma-action.org.uk Chemotherapy regimens for lymphoma Chemotherapy drugs for lymphoma are usually given as a ‘regimen’, a treatment plan that includes more than one type of drug. We have separate information about how chemotherapy works, the ways it’s given and its possible side effects. On this page What is a chemotherapy regimen? Why is chemotherapy given as a regimen? Common chemotherapy regimens for lymphoma Frequently asked questions What is a chemotherapy regimen? A chemotherapy regimen is a treatment plan that usually involves more than one drug. The regimen sets out: • the name of the drugs • the dose of each drug • how often you take the drugs • how the drugs are given • how long you take each drug for. Most regimens are given as a block of chemotherapy followed by a rest period to allow your body to recover. This is known as a ‘cycle’. Cycles often last between 2 and 4 weeks. A whole course of treatment can vary from several weeks to a number of months. Why is chemotherapy given as a regimen? Some chemotherapy drugs are given on their own. Examples include pixantrone and bendamustine. Often, however, a combination of chemotherapy drugs are given to treat lymphoma. Each drug works in a slightly different way to kill the lymphoma cells. For some types of lymphoma, this might mean that the drugs are able to kill more of the lymphoma than if you have them alone. You might be interested in our animation video that explains how chemotherapy works. -

Thames Valley Chemotherapy Regimens Lung Cancer
Thames Valley Thames Valley Chemotherapy Regimens Lung Cancer Thames Valley Notes from the editor These regimens are available on the Network website www.tvcn.org.uk. Any correspondence about the regimens should be addressed to: Sally Coutts, Cancer Pharmacist, Thames Valley email: [email protected] Tel: 01865 857158 to leave a message Acknowledgements These regimens have been compiled by the Network Pharmacy Group in collaboration with the Lung TSSG with key contributions from Dr Nick Bates, Consultant Oncologist, ORH Dr Paul Rogers, Consultant Oncologist, RBFT Dr Joss Adams, Consultant Oncologist, RBFT Sally Punter, formerly Oncology Pharmacist, ORH Sandra Harding Brown, formerly Specialist Principal Pharmacist Oncology, ORH Alison Ashman, formerly Lead Pharmacist Thames Valley Cancer Network © Thames Valley Cancer Network. All rights reserved. Not to be reproduced in whole or in part without the permission of the copyright owner. Network Chemotherapy Protocols – Lung Cancer 2 Thames Valley Thames Valley Chemotherapy Regimens Lung Cancer Network Chemotherapy regimens used in the management of Lung Cancer Date published: March 2015 Date of review: March 2017 Chemotherapy Regimens Name of protocol Indication Page List of amendments to this version 5 Notes 6 ACE Small Cell lung 7 CAV Small Cell lung 9 Carboplatin Etoposide Small cell lung 11 Cisplatin (75) Etoposide (100) Small cell lung 13 Cisplatin (60) Etoposide(120) Small cell lung 15 Topotecan oral Small cell lung 17 Afatnib Non small cell lung 19 Cisplatin (50) Etoposide (50) -

AC 60/600) 14 Day Followed by Paclitaxel (175) 14 Day Therapy (DD AC-T
NCCP Chemotherapy Regimen Dose Dense DOXOrubicin, Cyclophosphamide (AC 60/600) 14 day followed by PACLitaxel (175) 14 day Therapy (DD AC-T) Note: There is an option for DOXOrubicin, cyclophosphamide followed by weekly PACLitaxel (AC–T) therapy described in regimen NCCP00260. INDICATIONS FOR USE: Regimen Reimbursement INDICATION ICD10 Code Status Adjuvant Treatment of High Risk Node Negative or Node C50 00278a Hospital Positive Breast Cancer. Neoadjuvant Treatment of High Risk Node Negative or Node C50 00278b Hospital Positive Breast Cancer. TREATMENT: The starting dose of the drugs detailed below may be adjusted downward by the prescribing clinician, using their independent medical judgement, to consider each patients individual clinical circumstances. DOXOrubicin and cyclophosphamide are administered once every 14 days for four cycles (one cycle = 14 days) followed by PACLitaxel once every 14 days for 4 cycles (8weeks) to start 14 days after final cycle of DOXOrubicin and cyclophosphamide. G-CSF support (using standard or pegylated form) is required with all cycles. Facilities to treat anaphylaxis MUST be present when the chemotherapy is administered. Cycle 1-4: Admin. Day Drug Dose Route Diluent & Rate Cycle Order 1 1 DOXOrubicin 60mg/ m2 IV push N/A Every 14 days for 4 cycles 2 1 Cyclophosphamide 600mg/m2 IV infusion* 250ml 0.9% NaCl over 30min Every 14 days for 4 cycles * Cyclophosphamide may also be administered as an IV bolus over 5-10mins Lifetime cumulative dose of DOXOrubicin is 450mg/m2 In establishing the maximal cumulative dose of an anthracycline, consideration should be given to the risk factors outlined belowi and to the age of the patient. -
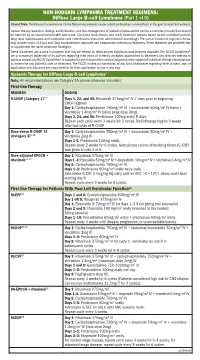
Diffuse Large B-Cell Lymphoma (Part 1 of 5)
NON-HODGKIN LYMPHOMA TREATMENT REGIMENS: Diffuse Large B-cell Lymphoma (Part 1 of 5) Clinical Trials: The National Comprehensive Cancer Network recommends cancer patient participation in clinical trials as the gold standard for treatment. Cancer therapy selection, dosing, administration, and the management of related adverse events can be a complex process that should be handled by an experienced health care team. Clinicians must choose and verify treatment options based on the individual patient; drug dose modifications and supportive care interventions should be administered accordingly. The cancer treatment regimens below may include both U.S. Food and Drug Administration-approved and unapproved indications/regimens. These regimens are provided only to supplement the latest treatment strategies. These Guidelines are a work in progress that may be refined as often as new significant data become available. The NCCN Guidelines® are a consensus statement of its authors regarding their views of currently accepted approaches to treatment. Any clinician seeking to apply or consult any NCCN Guidelines® is expected to use independent medical judgment in the context of individual clinical circumstances to determine any patient’s care or treatment. The NCCN makes no warranties of any kind whatsoever regarding their content, use, or application and disclaims any responsibility for their application or use in any way. Systemic Therapy for Diffuse Large B-cell Lymphoma1 Note: All recommendations are Category 2A unless otherwise indicated. First-line Therapy REGIMEN DOSING R-CHOP (Category 1)2–7 Days 1, 22, and 43: Rituximab 375mg/m2 IV 7 days prior to beginning CHOP regimen Day 1: Cyclophosphamide 750mg/m2 IV + doxorubicin 50mg/m2 IV bolus + vincristine 1.4mg/m2 IV bolus (max dose 2mg) Days 3, 24, and 45: Prednisone 100mg orally 5 days. -

The Cancer and Leukemia Group B Lymphoma Committee Bruce D
The Cancer and Leukemia Group B Lymphoma Committee Bruce D. Cheson1and George P. Canellos2 Abstract The malignant lymphomas include at least 30 entities that are distinct with respect to histology, immunology, genetics, clinical features, and outcome following therapy. The clinical behavior of these diseases ranges from indolent but generally incurable to aggressive and frequently fatal yet potentially curable with appropriate chemotherapy or chemotherapy-antibody regimens. Over the past 50 years, the Cancer and Leukemia Group B (CALGB) Lymphoma Committee has con- ducted a series of clinical trials that have contributed to an improvement in outcome for patients with a number of the more common lymphoma subtypes. The World Health Organization has classified approximately therapy alone. Although radiation therapy plays less of a role in 30 neoplastic diseases of the hematopoietic and lymphoid the current management of this subgroup of patients given the tissues (1). The Cancer and Leukemia Group B (CALGB) greater efficacy of contemporary chemotherapy regimens, this Lymphoma Committee highlight below clinical trials that have study provided important information about the use of resulted in improved patient outcome for the more frequent combined modality treatment at the time it was published. lymphoma subtypes. The mechlorethamine, vincristine, prednisone, and procar- bazine (MOPP) combination chemotherapy regimen provided the first evidence for cure in a substantial proportion of Hodgkin’s Lymphoma patients with stage III and IV Hodgkin’s lymphoma. Never- Hodgkin’s lymphoma represents one of the successes of theless, over the decades since the introduction of MOPP, modern oncology. Patients with limited stage disease were other regimens were developed to improve on the efficacy of traditionally treated with radiation therapy as the primary MOPP while attempting to reduce its toxicities, particularly modality. -

Weekly Chemotherapy with Cisplatin and Paclitaxel in Inoperable Non-Small Cell Lung Cancer: an Extended Phase II Study*
ANTICANCER RESEARCH 25: 4685-4692 (2005) Weekly Chemotherapy with Cisplatin and Paclitaxel in Inoperable Non-small Cell Lung Cancer: An Extended Phase II Study* DOMENICO FERRIGNO and GIANFRANCO BUCCHERI Struttura Complessa di Pneumologia, Azienda Ospedaliera "S. Croce e Carle", Cuneo, I-12100, Italy Abstract. Background: A phase II study of a (4). Patients with locally advanced (Stage III) or cisplatin/paclitaxel combination given on a weekly schedule in metastatic disease (Stage IV) (5) receive chemotherapy, the front-line treatment of non-small cell lung cancer since first-line platinum-based combinations improve (NSCLC) is reported. Patients and Methods: Treatment survival, palliate symptoms and ameliorate quality of life consisted of an intravenous infusion of cisplatin, 25 mg/m2, (6-9). Unfortunately, there is no consensus on which drug and paclitaxel, 80 mg/m2, every week. Chemotherapy was combination or treatment schedule should be continued until completion of a 22-week treatment plan, recommended in everyday practice (10). Randomized disease progression, persistent toxicity, or patient refusal. trials seem to support the use of two-drug combinations, Results: Seventy-nine patients entered the study. The median containing at least one new agent, such as vinorelbine, number of infusions per patient was 14 (range 0-22). The gemcitabine, or the taxanes (11). median dose-intensity was 75% of that projected. Toxicity was Paclitaxel was the first identified member of a new class generally acceptable, and never life-threatening. Seven of anticancer drugs known as the taxanes, which also complete responses (pathologically documented in 4 patients) includes docetaxel. Paclitaxel promotes the polymerization and 27 partial responses were observed for an overall response of tubulin, producing extraordinarily stable and rate of 43%.