Health Care Fraud
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Why Jackson's Man in the Mirror Never Could Make a “CHANGE!”
“I’m starting with the man in the mirror. I’m asking him to Why Jackson’s Man in the change his ways. No message could have been any clearer. If you want to make the world a better place take a look at Mirror never could make a yourself and make a change.” Then like a preacher he gets into it at the end of the song “CHANGE!” and starts saying “you’ve got to move…SHOME ON!” You gotta do something. The song was passionate and called James 1.19-27 | Mirrors Part 2 | April 10, 2016 people to change the world by changing themselves. That’s inspiring! Missions Night Tomorrow Night But it was flawed. The flaw was that it didn’t give any The greatest album of all time was released in 1987. The power to change. It called for change without talking about album was called “bad” and it was by a guy named Michael the power for lasting change. And so people got eXcited for Jackson. Yes, thriller sold twice as many copies as any other a while, but eventually even Jackson himself didn’t any album in history. But Bad ranks #10 all time in sales experience lasting change for the better in his own well and was just a better all around album. From that album documented life. came 5 consecutive #1 billboard songs. “I just can’t stop loving you,” “bad” “the way you make me feel” “dirty Diana” But he was on point about change. All change begins by and “The Man in the Mirror.” I don’t know if you need to looking in the mirror and seeing the real you. -

1911 June Acropolis
Whittier College Wardman Library Poet Commons Acropolis (Yearbook) Archives and Special Collections 6-1911 1911 June Acropolis Whittier College Follow this and additional works at: https://poetcommons.whittier.edu/acropolis ON WE HOLD IT UP TO YOU Our offer to make to order the best fitting suit, of the choicest materials and most perfect finish for a moderate amount of money. In thorough work- manship we challenge any tailor in town to beat us, and the materials, linings, etc., are guaranteeds to be of the best. And we have in our show- rooms at present, fabrics and patterns that cannot be duplicated at anything like our prices. We make the best $35.00 suits in Los Angeles City. SHIELDS & OPP 201-205 Delta Building 426 South Spring Street Los Angeles DW.B NICOLE CARL. ENTOLMANN FRED WALTER,JR. ODE PADS AND TALON PRESIDENT AND GEN'L MAR. SECRETARY, irJ '1 flrIrj(3 MAN U PACTU R 1 N .1 JrwFI I- R ,t%D WATCfl1 tI4IIt FACTORYAND SALESROOMSAT 217 S-SPRING ST., F MONIES;___________ SANDEr MAIN 9300 LOSANGFLES,CAL. ROME EXCHANGE 9900 The jno. R. King Citrus Nurseries WHOLESALE ONLY An excellent line of Washington Navels, Valencies and Eureka Lemons for sale. Specialty on contracts for stock to be delivered in season of 1911 and 1912 at very reasonable prices. CITRUS STOCK EXCLUSIVELY—NO SIDE LINES NORTH CITRUS AVE. NORTHMAGNOLIA AVE. Phone JNO. R. KING, Proprietor TRY THE Whittier Hdw. Co. for all your athletic game sup- plies. If they do not have it in stock they will order it. -

A Diary Manifesto for Oil Painters Amy Wing
RHYTHYM AND THE MONSTROUS: A DIARY MANIFESTO FOR OIL PAINTERS AMY WING-HANN WONG A THESIS SUBMITTED TO THE FACULTY OF GRADUATE STUDIES IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF FINE ARTS GRADUATE PROGRAM IN VISUAL ARTS YORK UNIVERSITY TORONTO, ONTARIO MAY 2015 © AMY WONG 2015 ABSTRACT I’M GOING TO TRY DESPARATELY TO FOLLOW OPRAH’S MEDIA TACTIC HERE. INTRO: what I am about to tell you BODY: TELL IT. END: summarize what I just told you. The invisible threads that form identity politics are especially messy today. Through the lens of a transnational/intersectional/feminist sensibility, my thesis paper and body of work weaves influences from both visual and music culture. Socio-political agency is explored through reconfiguration. Both thesis and artwork are informed by the organizational principles of collage logic - specifically through the contrast in texture and rhythm, and employing the notion of the monster as a harmony of incongruence. All in all, this is an account of the struggles of Diaspora Repping and artistic practice, and the dilemma of ensuing ‘rep sweats’. ii ACKNOWLEDGEMENT I would like to express sincere thanks to my power trio thesis committee - Janet Jones, Brandon Vickerd and Suzanne Carte for their incredible insight, feedback and support. I would like to thank all my mentors and colleagues throughout my artistic and academic career. Thank you to my mom Regina, my dad Paul, my sister Polly, my brother Chris. And my fabtabulous BFFs at home and abroad. Together we make up this electric web of equal parts craziness and unconditional love, and without this I would not be able to do what I do. -
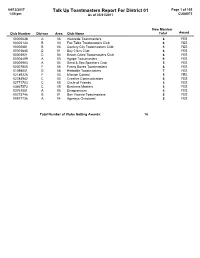
Talk up Toastmasters Report for District 01 Page 1 of 105 1:08 Pm As of 03/31/2017 CUS8073
04/13/2017 Talk Up Toastmasters Report For District 01 Page 1 of 105 1:08 pm As of 03/31/2017 CUS8073 New Member Club Number DivisonArea Club Name Total Award 00000638 A 06 Westside Toastmasters 8 YES 00002133 B 03 Fox Talkz Toastmasters Club 6 YES 00002681 B 03 Century City Toastmasters Club 5 YES 00003645 D 01 Bay Cities Club 6 YES 00003921 C 06 Beach Cities Toastmasters Club 6 YES 00004489 A 03 Agape Toastmasters 9 YES 00005983 A 04 Sand & Sea Speakers Club 5 YES 00007800 F 06 Funny Bones Toastmasters 6 YES 01098501 D 06 Herbalife Toastmasters 7 YES 02189226 F 03 Mission Control 5 YES 02284942 C 03 Creative Communicators 6 YES 02771703 C 05 Circle of Friends 5 YES 03667573 C 05 Business Masters 5 YES 03761051 A 05 Divapreneurs 5 YES 05273746 B 01 San Vicente Toastmasters 5 YES 05977736 A 04 Agensys Oncotoast 5 YES Total Number of Clubs Getting Awards: 16 04/13/2017 Talk Up Toastmasters Report For District 02 Page 2 of 105 1:08 pm As of 03/31/2017 CUS8073 New Member Club Number DivisonArea Club Name Total Award 00000304 E 54 Willows Voices Club 5 YES 00000822 E 53 Kirkland Eclectics Toastmasters Club 5 YES 00000832 D 43 West Seattle Toastmasters Club #832 6 YES 00007971 G 73 BlackRock Speaks Seattle 5 YES 00008864 F 61 Sammamish Speechmakers Toastmasters Club 6 YES 00009604 A 13 Burlington Better Speakers 5 YES 00009856 C 35 FNWL Club 5 YES 00009928 G 71 Husky Toastmasters Club 8 YES 00597395 F 65 Peak Speakers Toastmasters Club 5 YES 00935462 G 74 Amazon Toastmasters Club 10 YES 01445448 E 52 MicroToast 5 YES 01926934 C 35 Career Masters -

Scene I the Farragut Boat Club South Side of Chicago
1 SIXTEEN INCHES SIXTEEN INCH 2 CHARACTERS FARRAGUT BOAT CLUB EDWARD: White, architect, 30, Harvard graduate, exceedingly friendly, quick to take the lead in a situation. Has become a proud Chicagoan, despite early upbringing in New England area. JAMES: White, 27, businessman, Yale graduate, a tightly wound man, prides himself on making lucrative deals. Considers himself one of the rebuilders of Chicago after the Great Fire. JOSEPH: White, lawyer, 29, Yale graduate, congenial, sometimes a bit clueless (despite being a lawyer). GEORGE: White, 33, reporter, Harvard graduate, reserved demeanor because he likes to keep his ear open for new information. As a reporter has seen the redevelopment of the city first-hand after the Fire and sees the changes in demographics. It interests and worries him. WAVELAND SOFTBALL FIELD (Note: The character descriptions are written as if they’re being spoken by one of the characters themselves, but more like someone who knows them. Hopefully it gives a feel for the tone, the mindset of the main characters in the play. They are rude, profane, blunt-by-accident, but familiar enough with each other to say the things they say.) EDDIE: One of those run-of-the-mill Chicago Polish guys (HE can call himself a “Polack” but you probably shouldn’t.) Looks about 10 years older than he really is, so I’m guessing he’s, I don't know, about 42 or something? His hair’s already turning grey. Kinda got some muscles but not from working out…just that naturally strong shit, you know? Works for the city like his father and uncle. -

Talking up and Talking Down: the Power of Positive Speaking
Journal of Social Issues, Vol. 71, No. 4, 2015, pp. 834--846 doi: 10.1111/josi.12152 Talking Up and Talking Down: The Power of Positive Speaking ∗ Susan T. Fiske , Hilary Bergsieker, Vanessa Constantine, Cydney Dupree, Deborah S. Holoien, Nicolas Kervyn, Lisa Leslie, and Jill Swencionis Princeton University Consistent with Lewin’s legacy and SPSSI’s traditions, out work has focused on in- equality and power dynamics between people. Drawing on interpersonal positivity biases, stereotype content emphasizing perceived warmth and competence, and on the compensation effect (trading off warmth and competence), we study how people communicate, understand, and present themselves and others, especially across status divides. First, polite communicators omit negativity in describing individuals, especially stereotyped ones. Negativity omission creates innuendo (its absence implies the negative information), which allows stereotype to stag- nate. Listeners understand the innuendo and infer the negativity from its omission. Impression-managers understand this dynamic and use positive innuendo: They downplay one aspect (e.g., warmth or competence) to convey the other. Status determines which strategy people use: High-status speakers talk down (warmly), and low-status speakers talk up (competently). Cross-race interactions also show this dynamic. This creates dysfunctional inter-status interactions, the two people operating at crossed purposes. Winning an award named for Kurt Lewin –like my lifelong membership in the Society for the Psychological Study of Social Issues—expresses what it means to do science that tries to make the world a better place. Lewin himself worked on only some of SPSSI’s three Ps (prejudice, poverty, and peace), but he did work on power (group processes and leadership), relevant to our topic here. -

Activity Orientation in the Talk of Politicians, News Journalists And
Activity orientation in the talk of politicians, news journalists and audiences Paul Dickerson University College London Submitted for Doctorate of Philosophy 1999 1 Abstract The talk of politicians, news-joumalists and audiences has been relatively neglected in social psychology and media studies. Within these approaches talk has been ignored altogether, treated as a symptom of cognitive or ideological processes or employed simply as a tool to gain access to ‘inner’ ‘meaning making’ or ‘outer’ behaviour. This thesis explored a corpus of talk data from a discursive perspective in which the talk itself was the focus. It was argued that politicians and news-joumalists could in different ways be seen to orientate to the ‘truthfulness’ of what they say. Thus politicians’ were found to cite others to corroborate their claims, and new-joumalists through their exchange of utterances attended to the co-construction their ‘impartiality’ and ‘authoritativeness’. Politicians were also found to construct intent in terms of acting in ‘the national interest’ - this ‘repertoire’ could blame or exonerate self and others depending crucially on talk- context in which it was produced. Audiences’ talk about their identity and contrasts with others was also explored. Their talk was analysed not to uncover their ‘meaning- makings’ or behaviour but instead to discover the activity orientations of their talk and its sensitivity to the surrounding talk context. In this way the talk of politicians, news- joumalists and audiences was not seen as a symptom of some separate, ‘underlying’ phenomena of interest nor as a mere tool to access their ‘inner’ or ‘outer’ world - but rather it was the focus of study itself. -

So, We Are Going to Record So Let's Go Around and Introduce Yourself Just Real Quick
So, we are going to record so let's go around and introduce yourself just real quick. We're not going to use the whole hour today. I'm Carl Duley. I'm extension agricultural agent for Buffalo County. I've been teaching this class for 20 plus years, I guess. This is the first time we're doing it this way. So be a little patient. Hopefully everybody will do that. And we'll do the best we can under the circumstances. So let's just hear a little bit about you where you're from. If you live on a farm, what grade you're in, things like that. And I'll go on my list. Let's start with how about Gabe, Gabe Lindsay. I work-- live on a turkey farm and Gab you're really faint so you get closer to the mic or turn your mic up. I work on a turkey farm and yesterday, Gabe, we're sorry. Can anybody else hear Gabe? I can just barely. Yeah, Gabe, why don't you, if you go down on the bottom of your screen there's a by the microphone. If you click on the arrow, it'll have you where you can test out your your mic and see if we can't get a little more volume. We'll we'll move on then. How about Madeline? Introduce yourself. Oh, I'm Madeline. I'm going into eighth grade. And I technically don't live on a farm but I work on my grandpa's farm down the road. -

Targeting Teens Grades 6-12
Targeting Teens Grades 6-12 Lesson Summary Students analyze advertisements geared toward teenagers. Overview In this lesson, students will: Identify strategies (in print) that advertisers use to persuade teen consumers to buy their products Watch a video about how the media influences teen trends and consumerism (Optional) Time 1-3 hours (1 hour + 1-2 hours for optional activity) Background There is nothing accidental in an ad. It is never "just a picture." A team of marketing experts, including psychologists, puts a lot of time, thought, and money into commercial advertisements. Most often, products are pitched to make us believe that our perceived deficiencies can be overcome by buying a certain product: We will become better basketball players if we buy the right shoes, or we will Vocabulary have more friends if we use the right cell phone network. All of an Consumer Culture ad’s components are consciously created to try to make us think or Consumer Demographic feel a certain way, and to distract us from things that would deter us Advertising Strategies from buying that product, like the social or environmental costs Brand loyalty associated with it. Since everything comes from something Disposable Income originally found in nature (natural resources), every manufactured Entertainment Media product has an impact on the natural world. This means that buying Cool Hunters less stuff is not only good for our pocketbooks, it also helps protect nature. Cross-Promotion Advertisers use strategies that are meant to manipulate specific Materials consumer groups into wanting and buying more stuff. “Targeting 8 teen magazines Teens” is one such strategy. -

November 2008
Volume 08 Issue 03 The Bark The official newsletter of Greyhound Pets, Inc. Presidents Message Moira Corrigan, President Happy Holidays!!! It has been an exciting few months for GPI. On July 5th we moved into the Hern Greyt Works (our new kennel) and said good-bye to the Red Barn. Then on July 12th we brought our first load of new dogs into the Greyt Works kennel. We have three part-time employees to help us look after our pups. If you are at the kennel be sure to intro- duce yourself to them. And we have many new volunteers, as well as our terrific existing volunteers, help- ing us turn out the dogs and love on them. I want to thank all the folks that have come out for our work parties at the Hern Greyt Works. Your help has been invaluable in putting things together and getting the kennel up and running. And thank you to the volunteers who continue to help us maintain the kennel and the grounds. Our next load of dogs wasn’t scheduled to be delivered until October from Kansas, but on August 24th the Woodlands track in Kansas closed and the Kansas folks asked us if we could take our October load at the beginning of September. Our volunteers rallied and we were able to make enough space in the kennel to take in the load and help out the Kansas folks. Thank you!! Our September load brought us dog number 4900. That is over 4900 dogs we have brought into GPI since our inception in 1985. -
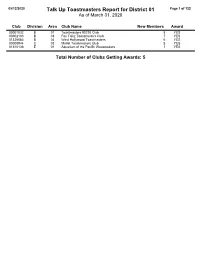
Talk up Toastmasters Report for District 01 Page 1 of 122 As of March 31, 2020
05/12/2020 Talk Up Toastmasters Report for District 01 Page 1 of 122 As of March 31, 2020 Club Division Area Club Name New Members Award 00001032 B 01 Toastmasters 90210 Club 8 YES 00002133 B 03 Fox Talkz Toastmasters Club 7 YES 01326683 B 04 West Hollywood Toastmasters 6 YES 00008944 C 03 Mattel Toastmasters Club 5 YES 01510136 E 01 Aquarium of the Pacific Wavemakers 7 YES Total Number of Clubs Getting Awards: 5 05/12/2020 Talk Up Toastmasters Report for District 02 Page 2 of 122 As of March 31, 2020 Club Division Area Club Name New Members Award 00935462 G 74 Amazon Toastmasters Roxanne Club 5 YES 00000540 B 25 Chamber Club 540 6 YES 00004077 E 52 Redmond Toasters Club 6 YES 00004365 E 55 Canyon Park Echoes 5 YES 00654507 D 44 SPOTLIGHT Toastmasters 6 YES 00000069 D 46 Renton Area PACCAR Toastmasters 6 YES 02967790 F 63 Downtown Bellevue Toastmasters 5 YES 05904243 F 65 Wing Talkers 5 YES 01587992 E 52 Redmond Nights 7 YES Total Number of Clubs Getting Awards: 9 05/12/2020 Talk Up Toastmasters Report for District 03 Page 3 of 122 As of March 31, 2020 Club Division Area Club Name New Members Award 07336142 T 04 You Can Toast It, We Can Help! 5 YES 00001576 N 04 Risk Takers Toastmasters Club 8 YES 00002083 T 01 Voice of Many Club 6 YES 00007153 Z 01 Noontime Toastmasters Club 5 YES 00004346 N 04 Good Evening Toastmasters 5 YES 00005059 V 02 USAA Eagles Toastmasters Club 5 YES 01445011 S 03 Queen Creek Toastmasters 5 YES Total Number of Clubs Getting Awards: 7 05/12/2020 Talk Up Toastmasters Report for District 04 Page 4 of 122 As of March -

Talk up Taihape May 2021
TALK UP TAIHAPE MAY 2021 SPRING FLING Will being held on Saturday the 18th of September 2021. We are ne- gotiating for a train visit which could result in a wonderful festi- val. Ideally, giving the opportunity to showcase Taihape and all its talents in the form of art, baking, preserving, sewing, knitting and woodwork. The winter period is a time to be creative. Holding joint stalls is a way of sharing the load. Gumboot Throwing and the Baby Animal Fair will be regular fea- tures and a new attraction a cultural food festival by our local cater- ers to showcase their talents and to give a TASTE of Taihape. We are hoping that our local clubs and organisations will climb aboard and take the opportunity to fundraise for their specific needs. Holding a Spring Fling is way to rid your winter blues and celebrate all things spring. Pania Winiata (Projects and Events Co-Ordinator) Taihape Community Development Trust E [email protected] P 06 388 1307 TALK UP TAIHAPE Advertisement Pricing and Deadline Voluntary Groups and Organisations, advertise your meetings and events in a classified advertisement (between 1/8 and 1/4 page) for FREE (subject to availability). 1 page (A5) $50 1/2 page $30 1/4 page $15 Advertising deadline Wednesday 2nd JUNE 2021 Publication and distribution Thursday 10th JUNE 2021 Email Sarah: [email protected] or call 06 388 1307. On line version available through www.taihape.co.nz 2 The upcoming Affordable Art Sale and Exhibition to be held at the Anglican Church hall on Huia Street is some- thing St.