Optogenetic Tractography for Anatomo-Functional Characterization of Cortico-Subcortical Neural Circuits in Non- Human Primates.” Scientific Reports, Vol
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cortex and Thalamus Lecture.Pptx
Cerebral Cortex and Thalamus Hyperbrain Ch 2 Monica Vetter, PhD January 24, 2013 Learning Objectives: • Anatomy of the lobes of the cortex • Relationship of thalamus to cortex • Layers and connectivity of the cortex • Vascular supply to cortex • Understand the location and function of hypothalamus and pituitary • Anatomy of the basal ganglia • Primary functions of the different lobes/ cortical regions – neurological findings 1 Types of Cortex • Sensory (Primary) • Motor (Primary) • Unimodal association • Multimodal association - necessary for language, reason, plan, imagine, create Note: • Gyri • Sulci • Fissures • Lobes 2 The Thalamus is highly interconnected with the cerebral cortex, and handles most information traveling to or from the cortex. “Specific thalamic Ignore nuclei” – have well- names of defined sensory or thalamic nuclei for motor functions now - A few Other nuclei have will more distributed reappear later function 3 Thalamus Midbrain Pons Limbic lobe = cingulate gyrus Structure of Neocortex (6 layers) white matter gray matter Pyramidal cells 4 Connectivity of neurons in different cortical layers Afferents = inputs Efferents = outputs (reciprocal) brainstem etc Eg. Motor – Eg. Sensory – more efferent more afferent output input Cortico- cortical From Thalamus To spinal cord, brainstem etc. To Thalamus Afferent and efferent connections to different ….Depending on whether they have more layers of cortex afferent or efferent connections 5 Different areas of cortex were defined by differences in layer thickness, and size and -
Lecture 12 Notes
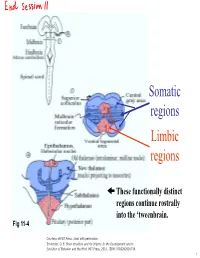
Somatic regions Limbic regions These functionally distinct regions continue rostrally into the ‘tweenbrain. Fig 11-4 Courtesy of MIT Press. Used with permission. Schneider, G. E. Brain structure and its Origins: In the Development and in Evolution of Behavior and the Mind. MIT Press, 2014. ISBN: 9780262026734. 1 Chapter 11, questions about the somatic regions: 4) There are motor neurons located in the midbrain. What movements do those motor neurons control? (These direct outputs of the midbrain are not a subject of much discussion in the chapter.) 5) At the base of the midbrain (ventral side) one finds a fiber bundle that shows great differences in relative size in different species. Give examples. What are the fibers called and where do they originate? 8) A decussating group of axons called the brachium conjunctivum also varies greatly in size in different species. It is largest in species with the largest neocortex but does not come from the neocortex. From which structure does it come? Where does it terminate? (Try to guess before you look it up.) 2 Motor neurons of the midbrain that control somatic muscles: the oculomotor nuclei of cranial nerves III and IV. At this level, the oculomotor nucleus of nerve III is present. Fibers from retina to Superior Colliculus Brachium of Inferior Colliculus (auditory pathway to thalamus, also to SC) Oculomotor nucleus Spinothalamic tract (somatosensory; some fibers terminate in SC) Medial lemniscus Cerebral peduncle: contains Red corticospinal + corticopontine fibers, + cortex to hindbrain fibers nucleus (n. ruber) Tectospinal tract Rubrospinal tract Courtesy of MIT Press. Used with permission. Schneider, G. -

Neuromodulation in Eating Disorders and Obesity: a Promising Way of Treatment?
Journal name: Neuropsychiatric Disease and Treatment Article Designation: Review Year: 2018 Volume: 14 Neuropsychiatric Disease and Treatment Dovepress Running head verso: Jáuregui-Lobera and Martínez-Quiñones Running head recto: Neuromodulation in eating disorders and obesity open access to scientific and medical research DOI: 180231 Open Access Full Text Article REVIEW Neuromodulation in eating disorders and obesity: a promising way of treatment? Ignacio Jáuregui-Lobera1 Abstract: Neuromodulation can affect the functioning of the central nervous system (CNS), José V Martínez-Quiñones2 and emotional/eating behavior is an exciting facet of that functioning. Therefore, it would be possible to offer an alternative (or complement) treatment to psychotropic medications and 1Department of Molecular Biology and Biochemical Engineering, different psychological and nutritional approaches to both eating disorders (EDs) and obe- University of Pablo de Olavide of sity. Although there are a number of publications in these areas, a systematic review has not Seville, Seville, Spain; 2Department of Neurosurgery, Mutua de been conducted to date. Abstracts, letters, conference reports, dissertations, and reviews were Accidentes de Zaragoza (Servicio de excluded. Clinical trials and controlled human clinical trials were filtered and included in this Neurocirugía), Zaragoza, Spain study. Articles included were based on the population suffering from anorexia nervosa, bulimia nervosa, binge ED, overweight, and obesity. No restrictions were placed on the sample size. Only trials investigating the effect of neuromodulation by means of deep brain stimulation (DBS), transcranial direct current stimulation (tDCS), and transcranial magnetic stimulation For personal use only. (TMS) were included. The following databases were used to conduct the search: MEDLINE/ PubMed, PsycINFO, PsycArticles, and Cochrane (Search Trials, CENTRAL). -

Dorsal “Thalamus”
Dorsal “Thalamus” Medical Neuroscience Dr. Wiegand The Diencephalon The Diencephalon InterthalamicInterthalamic adhesionadhesion ThalamusThalamus EpithalamusEpithalamus HypothalamusHypothalamus (Pineal(Pineal && Habenula)Habenula) PituitaryPituitary SubthalamusSubthalamus 1 The “Dorsal” Thalamus | Sensory integration nucleus – gateway to the cerebral cortex | Afferents from both rostral and caudal central nervous system structures | Efferents primarily to cerebral cortex via four principal “radiations” | Associated with motor, sensory, limbic and vegetative functions External medullary lamina Anterior n. 3rd Internal capsule Ventricle Medial n. Medial Lateral n. Internal capsule * Reticular n. Internal * Interthalamic adhesion medullary lamina 2 General Organization medialmedial nucleinuclei anterioranterior nuclei nuclei internalinternal medullarymedullary laminalamina laterallateral nuclei nuclei dorsaldorsal tiertier pulvinarpulvinar geniculategeniculate ventralventral tiertier bodiesbodies Frontal Section intralaminarintralaminar nucleinuclei reticularreticular nuclei nuclei 3rd Ventricle externalexternalexternalexternal medullarymedullary laminalamina internalinternal laminalamina medullarymedullary laminalamina 3 Thalamic Nuclei | Anterior | Lateral z Dorsal Tier • lateral dorsal • lateral posterior • pulvinar z Ventral Tier • ventral anterior • ventral lateral • ventral posterior (VLP & VPM) • posterior nucleus Thalamic Nuclei | Medial z medial/medial dorsal z midline nuclei | Pulvinar | Geniculate bodies | Reticular | Intralaminar -

Neuromodulation Symposium
MINNESOTA NEUROMODULATION SYMPOSIUM APRIL 14 - 15, 2016 THE COMMONS HOTEL MINNEAPOLIS, MN neuromodulation.umn.edu Dear Colleagues, Welcome to the 4th Annual Minnesota Neuromodulation Symposium. Neuromodulation is a rapidly-growing field, encompassing a wide spectrum of implantable and non-invasive technology-based approaches for the treatment of neurological and psychiatric disorders. It represents an important research nexus of basic and clinical neu- roscience, and of engineering sciences. It also represents an important industrial sector in the medical device industry with products already benefiting a number of patients. A collaborative effort by all stakeholders will be needed to further expedite the process of translating basic research discoveries into applied and clinical research, industrial research and development, and even manufacturing, which will result in a more immediate impact to healthcare. This symposium is aimed at bringing together basic scientists, engineers, clinicians, industrial practitioners, entrepreneurs, and policy makers to discuss challenges and opportunities in neuromodulation and neurotechnology. Advancing the field of neuromodulation represents challenges to developing engineering methodologies, understanding mechanisms of neuromodulation at cellular and system levels, translating research to treat patients, and closely integrating the regulatory process within the research and clinical environment. We are very pleased to address these challenges through our outstanding line-up of invited speakers that represent thought leaders from academia, industry, and government, whom over the next two days will review significant progress in neuromodulation and neurotechnology, providing a glimpse into the future of this fascinating field. We are also very pleased that the symposium has attracted a record number of scientific contributions from many institutions to be presented in the Poster Session. -

Diencephalon and Hypothalamus
Diencephalon and Hypothalamus Objectives: 1) To become familiar with the four major divisions of the diencephalon 2) To understand the major anatomical divisions and functions of the hypothalamus. 3) To appreciate the relationship of the hypothalamus to the pituitary gland Four Subdivisions of the Diencephalon: Epithalamus, Subthalamus Thalamus & Hypothalamus Epithalamus 1. Epithalamus — (“epi” means upon) the most dorsal part of the diencephalon; it forms a caplike covering over the thalamus. a. The smallest and oldest part of the diencephalon b. Composed of: pineal body, habenular nuclei and the caudal commissure c. Function: It is functionally and anatomically linked to the limbic system; implicated in a number of autonomic (ie. respiratory, cardio- vascular), endocrine (thyroid function) and reproductive (mating behavior; responsible for postpartum maternal behavior) functions. Melatonin is secreted by the pineal gland at night and is concerned with biological timing including sleep induction. 2. Subthalamus — (“sub” = below), located ventral to the thalamus and lateral to the hypothalamus (only present in mammals). a. Plays a role in the generation of rhythmic movements b. Recent work indicates that stimulation of the subthalamus in cats inhibits the micturition reflex and thus this nucleus may also be involved in neural control of micturition. c. Stimulation of the subthalamus provides the most effective treatment for late-stage Parkinson’s disease in humans. Subthalamus 3. Thalamus — largest component of the diencephalon a. comprised of a large number of nuclei; -->lateral geniculate (vision) and the medial geniculate (hearing). b. serves as the great sensory receiving area (receives sensory input from all sensory pathways except olfaction) and relays sensory information to the cerebral cortex. -

Diencephalon Diencephalon
Diencephalon Diencephalon • Thalamus dorsal thalamus • Hypothalamus pituitary gland • Epithalamus habenular nucleus and commissure pineal gland • Subthalamus ventral thalamus subthalamic nucleus (STN) field of Forel Diencephalon dorsal surface Diencephalon ventral surface Diencephalon Medial Surface THALAMUS Function of the Thalamus • Sensory relay – ALL sensory information (except smell) • Motor integration – Input from cortex, cerebellum and basal ganglia • Arousal – Part of reticular activating system • Pain modulation – All nociceptive information • Memory & behavior – Lesions are disruptive Classification of Thalamic Nuclei I. Lateral Nuclear Group II. Medial Nuclear Group III. Anterior Nuclear Group IV. Posterior Nuclear Group V. Metathalamic Nuclear Group VI. Intralaminar Nuclear Group VII. Thalamic Reticular Nucleus Classification of Thalamic Nuclei LATERAL NUCLEAR GROUP Ventral Nuclear Group Ventral Posterior Nucleus (VP) ventral posterolateral nucleus (VPL) ventral posteromedial nucleus (VPM) Input to the Thalamus Sensory relay - Ventral posterior group all sensation from body and head, including pain Projections from the Thalamus Sensory relay Ventral posterior group all sensation from body and head, including pain LATERAL NUCLEAR GROUP Ventral Lateral Nucleus Ventral Anterior Nucleus Input to the Thalamus Motor control and integration Projections from the Thalamus Motor control and integration LATERAL NUCLEAR GROUP Prefrontal SMA MI, PM SI Ventral Nuclear Group SNr TTT GPi Cbll ML, STT Lateral Dorsal Nuclear Group Lateral -

The Human Basal Ganglia Mediate the Interplay Between Reactive and Proactive Control of Response Through Both Motor Inhibition and Sensory Modulation
brain sciences Article The Human Basal Ganglia Mediate the Interplay between Reactive and Proactive Control of Response through Both Motor Inhibition and Sensory Modulation Marion Criaud 1,*, Jean-Luc Anton 2, Bruno Nazarian 2, Marieke Longcamp 3, Elise Metereau 4, Philippe Boulinguez 5,6,7,8,† and Bénédicte Ballanger 5,6,7,8,† 1 Institute of Psychiatry Psychology & Neuroscience, Department Child & Adolescent Psychiatry, Kings College London, London SE24 9QR, UK 2 Centre IRM-INT@CERIMED, Institut de Neurosciences de la Timone, CNRS UMR7289 & Aix-Marseille Université, 13005 Marseille, France; [email protected] (J.-L.A.); [email protected] (B.N.) 3 Laboratoire de Neurosciences Cognitives, CNRS UMR 7291 & Aix-Marseille Université, 13005 Marseille, France; [email protected] 4 Hôpital Neurologique Pierre Wertheimer, Service de Neurologie C, Centre Expert Parkinson, Hospices Civils de Lyon, 69677 Bron, France; [email protected] 5 Université de Lyon, 69622 Lyon, France; [email protected] (P.B.); [email protected] (B.B.) 6 Université Claude Bernard Lyon 1, 69100 Villeurbanne, France 7 INSERM, U 1028, Lyon Neuroscience Research Center, 69000 Lyon, France 8 CNRS, UMR 5292, Lyon Neuroscience Research Center, 69000 Lyon, France Citation: Criaud, M.; Anton, J.-L.; * Correspondence: [email protected] Nazarian, B.; Longcamp, M.; † Co-last authors. Metereau, E.; Boulinguez, P.; Ballanger, B. The Human Basal Abstract: The basal ganglia (BG) have long been known for contributing to the regulation of mo- Ganglia Mediate the Interplay tor behaviour by means of a complex interplay between tonic and phasic inhibitory mechanisms. -

The Dorsal Column Nuclei Neuroanatomy Reveals a Complex Sensorimotor Integration and Distribution Hub
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 8 November 2019 doi:10.20944/preprints201911.0084.v1 The Dorsal Column Nuclei Neuroanatomy Reveals A Complex Sensorimotor Integration and Distribution Hub Alastair J Loutit1,2, Richard M Vickery1, and Jason R Potas1,2 * 1School of Medical Sciences, UNSW Sydney, Sydney, New South Wales, 2052, Australia 2The Eccles Institute of Neuroscience, John Curtin School of Medical Research, Australian National University, Canberra, Australian Capital Territory, 2601, Australia * Correspondence: Dr Jason R Potas [email protected] Number of words (excluding references, abstract and abbreviations): 14,490 Number of figures: 6 Number of Tables: 1 Number of supplementary figures: 0 Keywords: brainstem sensory nuclei; somatosensation; secondary afferents; posterior column Abstract The dorsal column nuclei (DCN) are organised by both somatotopy and modality, and have a diverse range of afferent inputs and projection targets. The functional organisation and connectivity of the DCN implicate them in a variety of sensorimotor functions, beyond their commonly accepted role in processing and transmitting somatosensory information to the thalamus, yet this is largely underappreciated in the literature. In this review, we examine the morphology, organisation, and connectivity of the DCN and their associated nuclei, to improve understanding of their sensorimotor functions. First, we briefly discuss the receptors, afferent fibres, and pathways involved in conveying tactile and proprioceptive information to the DCN. Next, we review the modality and somatotopic arrangements of the constituents of the dorsal column nuclei complex (DCN-complex), which includes the gracile, cuneate, external cuneate, X, and Z nuclei, and Bischoff’s nucleus. Finally, we examine and discuss the functional implications of the myriad of DCN-complex projection targets throughout the midbrain, and hindbrain, in addition to their modulatory inputs from the cortex. -

Essential Neuromodulation This Page Intentionally Left Blank Essential Neuromodulation
Essential Neuromodulation This page intentionally left blank Essential Neuromodulation Jeffrey E. Arle Director, Functional Neurosurgery and Research, Department of Neurosurgery Lahey Clinic Burlington, MA Associate Professor of Neurosurgery Tufts University School of Medicine, Boston, MA Jay L. Shils Director of Intraoperative Monitoring, Dept of Neurosurgery Lahey Clinic Burlington, MA AMSTERDAM • BOSTON • HEIDELBERG • LONDON NEW YORK • OXFORD • PARIS • SAN DIEGO SAN FRANCISCO • SINGAPORE • SYDNEY • TOKYO Academic Press is an Imprint of Elsevier Academic Press is an imprint of Elsevier 32 Jamestown Road, London NW1 7BY, UK 30 Corporate Drive, Suite 400, Burlington, MA 01803, USA 525 B Street, Suite 1800, San Diego, CA 92101-4495, USA First edition 2011 Copyright © 2011 Elsevier Inc. All rights reserved No part of this publication may be reproduced, stored in a retrieval system or transmitted in any form or by any means electronic, mechanical, photocopying, recording or otherwise without the prior written permission of the publisher Permissions may be sought directly from Elsevier's Science & Technology Rights Department in Oxford, UK: phone ( + 44) (0) 1865 843830; fax ( +44) (0) 1865 853333; email: [email protected]. Alternatively, visit the Science and Technology Books website at www.elsevierdirect.com/rights for further information Notice No responsibility is assumed by the publisher for any injury and/or damage to persons or property as a matter of products liability, negligence or otherwise, or from any use or operation of -

Localization of an Experimental Hypo- Thalamic and Midbrain Syndrome Simulating Sleep
LOCALIZATION OF AN EXPERIMENTAL HYPO- THALAMIC AND MIDBRAIN SYNDROME SIMULATING SLEEP EMMA H. COLLINS’ Laboratory of Comprutive Neurology, Depcartntent of Amtomy, the Univeu%ty of Michigan Medical School, Ann Arbor AND Department of Anatomy, the University of Illinois Medical School, Chicago FIF”F,EN FIGURES The origin and significance of sleep have been a source of speculation throughout the centuries, and the problem of abnormal sleep as a disease entity has long aroused the curiosity of medical men. It was only half a century ago, however, that Mauthner (lS90), after studying clinical cases of Nona and Wernicke’s disease, postulated a specific locality in the brain as a critical source for sleep regulation. This center he designated the area of transition between the mid- brain and the diencephalon. More recently, von Economo (’29, ’30, ’31), from his study of encephalitis lethargica during the Vienna epidemic of 1916-17 and 1920-21, came to conclusions similar to those of Mauthner and proposed certain rostro-caudal boundaries to delimit the center : the most caudal limit being immediately rostra1 to the oculomotor iiucleus and the frontal limit reach- ing as far as the region of the striatum. ‘This study was initiated as research for a dissertation, conducted under the direction of Dr. Elizabeth Caroline Crosby, in partial fulfillment of the require- ments for the degree of Doctor of Philosophy from the Rackham School of Gradu- ate Studies of the University of Michigan. The monkeys and laboratory facilities were made available through grants from the Brower and Am Research Fund and from the Alphonso Morton Clover Research Fund, by the University of Midi- gan Medical School. -

Diencephalon August 30, 2011 G
Neuroscience Diencephalon August 30, 2011 G. Gruener, MD, MBA DIENCEPHALON Date: August 30, 2011 – 9:30 AM Reading Assignment: Diencephalon lecture notes – Gruener Diencephalon presentation – Gruener Medical Neurobiology by Mason – “263-312” KEY CONCEPTS & LEARNING OBJECTIVES 1. After attending lecture and studying the assigned material you will be able to: a.) Outline the major organizational divisions of the thalamus. b.) Describe the major functional divisions of the thalamus c.) List the major interconnections between thalamus and cerebral cortex (four thalamic peduncles) d.) Be able to identify the thalamus and its relationships to the internal capsule, basal ganglia and third ventricle 2. After attending lecture and studying the assigned material you will be able to: a.) Identify the specific (or relay) nuclei of the thalamus, source of their afferents and which ones project to: i. Prefrontal cortex ii. Primary motor cortex iii. Somatic sensory cortex iv. Primary visual cortex v. Primary auditory cortex b.) Name the association nuclei of the thalamus and define their role/function c.) Name the non-specific thalamic nuclei of the thalamus and define their role/function 3. After attending lecture and studying the assigned material you will be able to: a.) Describe the clinical features seen with thalamic lesions b.) Describe the blood supply to the thalamus 4. After review of the clinical case presentations in the small groups you will be able to: a.) Suggest a site of dysfunction that will explain the signs and symptoms b.) Identify the expected site of an abnormality on an MRI scan of the brain c.) Start to develop three potential etiologies (appropriate to the patients’ clinical scenario, course and medical history) that would explain their presentation.