Leaf Arrangements Are Invalid in the Taxonomy of Orchid Species
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Germination, Vegetative and Flowering Behavior of Balsam (Impatiens
International Journal of Environment, Agriculture and Biotechnology (IJEAB) Vol-3, Issue -4, Jul-Aug- 2018 http://dx.doi.org/10.22161/ijeab/3.4.38 ISSN: 2456-1878 Germination, vegetative and flowering behavior of Balsam (Impatiens balsamina L.) in response to natural photoperiods Muhammad Aslam Baloch1, Tanveer Fatima Miano*1, Niaz Ahmed Wahocho1, Naheed Akhtar Talpur2, Abdul Qadir Gola1 1Department of Horticulture, Sindh Agriculture University Tandojam, Pakistan 2Department of Soil Science, Sindh Agriculture University Tandojam, Pakistan Corresponding Author: Dr. Tanveer Fatima Miano*, Associate Professor Corresponding Author Email: [email protected] Abstract— A lack of application of photoperiod and light content (46.79 SPAD) was recorded from NP4= 9 hrs intensity to manipulate the growth of current spring (8:00 am-5:00 pm) as compared to seed germination annuals has, in part, been due to the lack of information (63.88%), germination index (0.66 gi) plant height (14.92 identifying the photoperiodic and light intensities cm), leaves plant-1 (48.16), days to 1st flower (45.96), requirements of various species. Present pot experiment flowers plant-1 (5.00), days to flower persistence (11.16), was carried out at Horticulture Garden, Department of weight of single flower (0.62 g), chlorophyll content (36.46 Horticulture, Sindh Agriculture University Tandojam, SPAD) was recorded from NP1= Control (Normal day during spring 2017, which was laid out in a three length). replicated Complete Randomized Design (CRD). Two Keywords— flowering behaviour, natural photoperiods, varieties of balsam (V1= Tom Thumb, V2 = Double Complete Randomized Design. Camcellia) were studied under NP1= Control (Normal day length), NP2=3 hrs (8:00 am- 11:00 am), NP3= 6 hrs (8:00 I. -

The Vascular Plants of Massachusetts
The Vascular Plants of Massachusetts: The Vascular Plants of Massachusetts: A County Checklist • First Revision Melissa Dow Cullina, Bryan Connolly, Bruce Sorrie and Paul Somers Somers Bruce Sorrie and Paul Connolly, Bryan Cullina, Melissa Dow Revision • First A County Checklist Plants of Massachusetts: Vascular The A County Checklist First Revision Melissa Dow Cullina, Bryan Connolly, Bruce Sorrie and Paul Somers Massachusetts Natural Heritage & Endangered Species Program Massachusetts Division of Fisheries and Wildlife Natural Heritage & Endangered Species Program The Natural Heritage & Endangered Species Program (NHESP), part of the Massachusetts Division of Fisheries and Wildlife, is one of the programs forming the Natural Heritage network. NHESP is responsible for the conservation and protection of hundreds of species that are not hunted, fished, trapped, or commercially harvested in the state. The Program's highest priority is protecting the 176 species of vertebrate and invertebrate animals and 259 species of native plants that are officially listed as Endangered, Threatened or of Special Concern in Massachusetts. Endangered species conservation in Massachusetts depends on you! A major source of funding for the protection of rare and endangered species comes from voluntary donations on state income tax forms. Contributions go to the Natural Heritage & Endangered Species Fund, which provides a portion of the operating budget for the Natural Heritage & Endangered Species Program. NHESP protects rare species through biological inventory, -

Epipactis Tremolsii Seed Diversity in Two Close but Extremely Different Populations: Just a Case of Intraspecific Variability?
plants Article Epipactis tremolsii Seed Diversity in Two Close but Extremely Different Populations: Just a Case of Intraspecific Variability? 1 1, , 1 2 Antonio De Agostini , Pierluigi Cortis * y , Annalena Cogoni , Roberta Gargiulo and Giuseppe Fenu 1 1 Department of Life and Environmental Sciences, University of Cagliari, Via Sant’Ignazio da Laconi 13, 09123 Cagliari, CA, Italy; [email protected] (A.D.A.); [email protected] (A.C.); [email protected] (G.F.) 2 Royal Botanic Gardens, Kew Richmond, Surrey TW9 3DS, UK; [email protected] * Correspondence: [email protected] Shared first co-authorship. y Received: 9 November 2020; Accepted: 20 November 2020; Published: 23 November 2020 Abstract: Analysis of the seed morphology is a widely used approach in ecological and taxonomic studies. In this context, intraspecific variability with respect to seed morphology (size, weight, and density) was assessed in two close Epipactis tremolsii Pau. populations sharing the same ecological conditions, except for the soil pollution distinguishing one of them. Larger and heavier seeds were found in plants growing on the heavy metal polluted site, while no differences in seed density were detected between seeds produced by plants growing on the contaminated and the control site. Moreover, seed coats and embryos varying together in their dimensions were described in the control population, while coats varying in their size independently from embryos were described in plants growing on the polluted site. Seeds from the two studied populations significantly differed in several parameters suggesting that intraspecific seed variability occurred in the case study. Keywords: Epipactis; intraspecific variability; seed ecology; seed morphometry; heavy metals 1. -

Articles & Book Reviews
Newsletter of the Colorado Native Plant Society ARTICLES & BOOK REVIEWS Marr Steinkamp Research: Pollination Biology of the Stream Orchid Alpine Cushion Plants in New Zealand Interview with Barbara Fahey, Native Plant Master® Program Founder Conservation Corner: White River Beardtongue How Lupines Talk to Bees Volume 38, No. 2 Summer 2014 Aquilegia: Newsletter of the Colorado Native Plant Society Dedicated to furthering the knowledge, appreciation, and conservation of native plants and habitats of Colorado through education, stewardship, and advocacy Volume 38 Number 2 Summer 2014 ISSN 2161-7317 (Online) - ISSN 2162-0865 (Print) Inside this issue News & Announcements................................................................................................ 3 Field Trips........................................................................................................................6 Articles Marr/Steinkamp Research: Pollination Biology of Epipactis gigantea........................9 How Lupines Talk to Bees...........................................................................................11 The Other Down Under: Exploring Alpine Cushion Plants in New Zealand...........14 The Native Plant Master® Program: An Interview with Barbara Fahey.....................16 Conservation Corner: White River Beardtongue......................................................... 13 Book & Media Reviews, Song........................................................................................19 Calendar...................................................................................................................... -

GENOME EVOLUTION in MONOCOTS a Dissertation
GENOME EVOLUTION IN MONOCOTS A Dissertation Presented to The Faculty of the Graduate School At the University of Missouri In Partial Fulfillment Of the Requirements for the Degree Doctor of Philosophy By Kate L. Hertweck Dr. J. Chris Pires, Dissertation Advisor JULY 2011 The undersigned, appointed by the dean of the Graduate School, have examined the dissertation entitled GENOME EVOLUTION IN MONOCOTS Presented by Kate L. Hertweck A candidate for the degree of Doctor of Philosophy And hereby certify that, in their opinion, it is worthy of acceptance. Dr. J. Chris Pires Dr. Lori Eggert Dr. Candace Galen Dr. Rose‐Marie Muzika ACKNOWLEDGEMENTS I am indebted to many people for their assistance during the course of my graduate education. I would not have derived such a keen understanding of the learning process without the tutelage of Dr. Sandi Abell. Members of the Pires lab provided prolific support in improving lab techniques, computational analysis, greenhouse maintenance, and writing support. Team Monocot, including Dr. Mike Kinney, Dr. Roxi Steele, and Erica Wheeler were particularly helpful, but other lab members working on Brassicaceae (Dr. Zhiyong Xiong, Dr. Maqsood Rehman, Pat Edger, Tatiana Arias, Dustin Mayfield) all provided vital support as well. I am also grateful for the support of a high school student, Cady Anderson, and an undergraduate, Tori Docktor, for their assistance in laboratory procedures. Many people, scientist and otherwise, helped with field collections: Dr. Travis Columbus, Hester Bell, Doug and Judy McGoon, Julie Ketner, Katy Klymus, and William Alexander. Many thanks to Barb Sonderman for taking care of my greenhouse collection of many odd plants brought back from the field. -

Impatiens Glandulifera
NOBANIS – Invasive Alien Species Fact Sheet Impatiens glandulifera Author of this fact sheet: Harry Helmisaari, SYKE (Finnish Environment Institute), P.O. Box 140, FIN-00251 Helsinki, Finland, Phone + 358 20 490 2748, E-mail: [email protected] Bibliographical reference – how to cite this fact sheet: Helmisaari, H. (2010): NOBANIS – Invasive Alien Species Fact Sheet – Impatiens glandulifera. – From: Online Database of the European Network on Invasive Alien Species – NOBANIS www.nobanis.org, Date of access x/x/201x. Species description Scientific name: Impatiens glandulifera Royle (Balsaminaceae). Synonyms: Impatiens roylei Walpers. Common names: Himalayan balsam, Indian balsam, Policeman's Helmet (GB), Drüsiges Springkraut, Indisches Springkraut (DE), kæmpe-balsamin (DK), verev lemmalts (EE), jättipalsami (FI), risalísa (IS), bitinė sprigė (LT), puķu sprigane (LV), Reuzenbalsemien (NL), kjempespringfrø (NO), Niecierpek gruczolowaty, Niecierpek himalajski (PL), недотрога железконосная (RU), jättebalsamin (SE). Fig. 1 and 2. Impatiens glandulifera in an Alnus stand in Helsinki, Finland, and close-up of the seed capsules, photos by Terhi Ryttäri and Harry Helmisaari. Fig. 3 and 4. White and red flowers of Impatiens glandulifera, photos by Harry Helmisaari. Species identification Impatiens glandulifera is a tall annual with a smooth, usually hollow and jointed stem, which is easily broken (figs. 1-4). The stem can reach a height of 3 m and its diameter can be up to several centimetres. The leaves are opposite or in whorls of 3, glabrous, lanceolate to elliptical, 5-18 cm long and 2.5-7 cm wide. The inflorescences are racemes of 2-14 flowers that are 25-40 mm long. Flowers are zygomorphic, their lowest sepal forming a sac that ends in a straight spur. -
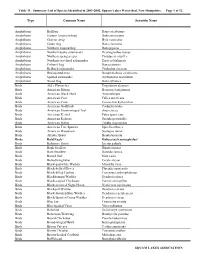
Summary Data
Table 11. Summary List of Species Identified in 2001-2002, Squam Lakes Watershed, New Hampshire. Page 1 of 12 Type Common Name Scientific Name Amphibians Bullfrog Rana catesbeiana Amphibians Eastern American toad Bufo americanus Amphibians Gray treefrog Hyla versicolor Amphibians Green frog Rana clamitans Amphibians Northern leopard frog Rana pipiens Amphibians Northern dusky salamander Desmognathus fuscus Amphibians Northern spring peeper Pseudacris crucifer Amphibians Northern two-lined salamander Eurycea bislineata Amphibians Pickerel frog Rana palustris Amphibians Redback salamander Plethodon cinereus Amphibians Red-spotted newt Notophthalmus viridescens Amphibians Spotted salamander Ambystoma maculatum Amphibians Wood frog Rana sylvatica Birds Alder Flycatcher Empidonax alnorum Birds American Bittern Botaurus lentiginosus Birds American Black Duck Anas rubripes Birds American Coot Fulica americana Birds American Crow Corvus brachyrhynchos Birds American Goldfinch Carduelis tristis Birds American Green-winged Teal Anas crecca Birds American Kestrel Falco sparverius Birds American Redstart Setophaga ruticilla Birds American Robin Turdus migratorius Birds American Tree Sparrow Spizella arborea Birds American Woodcock Scolopax minor Birds Atlantic Brant Branta bernicla Birds Bald Eagle* Haliaeetus leucocephalus* Birds Baltimore Oriole Icterus galbula Birds Bank Swallow Riparia riparia Birds Barn Swallow Hirundo rustica Birds Barred Owl Strix varia Birds Belted Kingfisher Ceryle alcyon Birds Black-and-white Warbler Mniotilta varia Birds -

Studies in the British Epipaci'is Vii. Seed Dimensions and Root Diameters
STUDIES IN THE BRITISH EPIPACI'IS VII. SEED DIMENSIONS AND ROOT DIAMETERS By DONALD P. YOUNG ABsTRACT Seed-dimensions and root-diameters are characters of some taxonomic significance in Epipactis species. Preliminary data on these measurements is given in the tables. Epipactis are notoriously variable plants, and it is not surprising that their quantitative characters show a wide amplitude and much overlapping between species. The data which follows indicates that the dimensions of the roots and seeds are more absolute characters and of taxonomic significance. It is not the result of a serious biometric study, but simply arose from examination of material that happened to be available. It is presented as an indication of where properly designed studies might be profitable. SEED DIMENSIONS The size of seeds is well known to be almost independent of the size and vigour of the parent plant. Dymes (1921, 1923) has shown that seed morphology is sometimes a useful taxonomic character in the Orchidaceae. Table 1 gives the dimensions of the seeds of all species of Epipactis, Section Epipactis, of Europe and the Mediterranean region, including five non-British species. Samples were mounted in Canada balsam, and measurements were made by means of a micrometer eyepiece on ten seeds taken at random (but rejecting twisted or undeveloped ones). On the basis of size and shape of the seeds, the species fall mainly into two groups : (i) E. purpura ta, E. microphylla, E. leptochila, and the three glabrous-stemmed species E. phyllanthes, E. con/usa, and E. persica, with large seeds 1·15-1·3 mm. -

Phylogeny, Character Evolution and the Systematics of Psilochilus (Triphoreae)
THE PRIMITIVE EPIDENDROIDEAE (ORCHIDACEAE): PHYLOGENY, CHARACTER EVOLUTION AND THE SYSTEMATICS OF PSILOCHILUS (TRIPHOREAE) A Dissertation Presented in Partial Fulfillment of the Requirements for The Degree Doctor of Philosophy in the Graduate School of the Ohio State University By Erik Paul Rothacker, M.Sc. ***** The Ohio State University 2007 Doctoral Dissertation Committee: Approved by Dr. John V. Freudenstein, Adviser Dr. John Wenzel ________________________________ Dr. Andrea Wolfe Adviser Evolution, Ecology and Organismal Biology Graduate Program COPYRIGHT ERIK PAUL ROTHACKER 2007 ABSTRACT Considering the significance of the basal Epidendroideae in understanding patterns of morphological evolution within the subfamily, it is surprising that no fully resolved hypothesis of historical relationships has been presented for these orchids. This is the first study to improve both taxon and character sampling. The phylogenetic study of the basal Epidendroideae consisted of two components, molecular and morphological. A molecular phylogeny using three loci representing each of the plant genomes including gap characters is presented for the basal Epidendroideae. Here we find Neottieae sister to Palmorchis at the base of the Epidendroideae, followed by Triphoreae. Tropidieae and Sobralieae form a clade, however the relationship between these, Nervilieae and the advanced Epidendroids has not been resolved. A morphological matrix of 40 taxa and 30 characters was constructed and a phylogenetic analysis was performed. The results support many of the traditional views of tribal composition, but do not fully resolve relationships among many of the tribes. A robust hypothesis of relationships is presented based on the results of a total evidence analysis using three molecular loci, gap characters and morphology. Palmorchis is placed at the base of the tree, sister to Neottieae, followed successively by Triphoreae sister to Epipogium, then Sobralieae. -

Epipactis Gigantea Dougl
Epipactis gigantea Dougl. ex Hook. (stream orchid): A Technical Conservation Assessment Prepared for the USDA Forest Service, Rocky Mountain Region, Species Conservation Project March 20, 2006 Joe Rocchio, Maggie March, and David G. Anderson Colorado Natural Heritage Program Colorado State University Fort Collins, CO Peer Review Administered by Center for Plant Conservation Rocchio, J., M. March, and D.G. Anderson. (2006, March 20). Epipactis gigantea Dougl. ex Hook. (stream orchid): a technical conservation assessment. [Online]. USDA Forest Service, Rocky Mountain Region. Available: http: //www.fs.fed.us/r2/projects/scp/assessments/epipactisgigantea.pdf [date of access]. ACKNOWLEDGMENTS This research was greatly facilitated by the helpfulness and generosity of many experts, particularly Bonnie Heidel, Beth Burkhart, Leslie Stewart, Jim Ferguson, Peggy Lyon, Sarah Brinton, Jennifer Whipple, and Janet Coles. Their interest in the project, valuable insight, depth of experience, and time spent answering questions were extremely valuable and crucial to the project. Nan Lederer (COLO), Ron Hartman, Ernie Nelson, Joy Handley (RM), and Michelle Szumlinski (SJNM) all provided assistance and specimen labels from their institutions. Annette Miller provided information for the report on seed storage status. Jane Nusbaum, Mary Olivas, and Barbara Brayfield provided crucial financial oversight. Shannon Gilpin assisted with literature acquisition. Many thanks to Beth Burkhart, Janet Coles, and two anonymous reviewers whose invaluable suggestions and insight greatly improved the quality of this manuscript. AUTHORS’ BIOGRAPHIES Joe Rocchio is a wetland ecologist with the Colorado Natural Heritage Program where his work has included survey and assessment of biologically significant wetlands throughout Colorado since 1999. Currently, he is developing bioassessment tools to assess the floristic integrity of Colorado wetlands. -

Landscaping Near Black Walnut Trees
Selecting juglone-tolerant plants Landscaping Near Black Walnut Trees Black walnut trees (Juglans nigra) can be very attractive in the home landscape when grown as shade trees, reaching a potential height of 100 feet. The walnuts they produce are a food source for squirrels, other wildlife and people as well. However, whether a black walnut tree already exists on your property or you are considering planting one, be aware that black walnuts produce juglone. This is a natural but toxic chemical they produce to reduce competition for resources from other plants. This natural self-defense mechanism can be harmful to nearby plants causing “walnut wilt.” Having a walnut tree in your landscape, however, certainly does not mean the landscape will be barren. Not all plants are sensitive to juglone. Many trees, vines, shrubs, ground covers, annuals and perennials will grow and even thrive in close proximity to a walnut tree. Production and Effect of Juglone Toxicity Juglone, which occurs in all parts of the black walnut tree, can affect other plants by several means: Stems Through root contact Leaves Through leakage or decay in the soil Through falling and decaying leaves When rain leaches and drips juglone from leaves Nuts and hulls and branches onto plants below. Juglone is most concentrated in the buds, nut hulls and All parts of the black walnut tree produce roots and, to a lesser degree, in leaves and stems. Plants toxic juglone to varying degrees. located beneath the canopy of walnut trees are most at risk. In general, the toxic zone around a mature walnut tree is within 50 to 60 feet of the trunk, but can extend to 80 feet. -

16-21 Review Article Impatiens Balsamina: an Overview
Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2015, 7(9):16-21 ISSN : 0975-7384 Review Article CODEN(USA) : JCPRC5 Impatiens balsamina: An overview Meenu B., Neeraja E. D., Greeshma Rejimon and Alexeyena Varghese* Department of Pharmacognosy, Amrita School of Pharmacy, Amrita Viswavidhyapeetham University, AIMS Health Sciences Campus, Kochi, Kerala, India _____________________________________________________________________________________________ ABSTRACT Impatiens balsamina belongs to the family balsaminaceae, is an annual plant commonly known as ‘garden balsam’ or ‘rose balsam’. This plant is native to southern Asia in India. I. balsamina is used in traditional methods such as Ayurveda, Unani and Siddha for various diseases and physiological conditions such as Jaundice, Corns, Snake bite etc. Phytochemical studies revealed the presence of many valuable compounds like naphthoquinones, coumarins, phenolic acids, flavonoids, anthocyanidins and steroids. The different parts of the plants like leaves, stem juice, flower are used in different places. I. balsamina have been reported to have various pharmacological activities such as antibacterial, antimicrobial, antifungal, analgesic, anti-inflammatory, antioxidant, antipruritic effects. The current review summarizes published information about the ethanopharmacology, chemical constituents, biological activities and toxicological study I.balsamina. The present review summarizes all the research work carried out on this plant in order to provide updated information