304601.Chp:Corel VENTURA
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Anatomy of the Temporal Lobe
Hindawi Publishing Corporation Epilepsy Research and Treatment Volume 2012, Article ID 176157, 12 pages doi:10.1155/2012/176157 Review Article AnatomyoftheTemporalLobe J. A. Kiernan Department of Anatomy and Cell Biology, The University of Western Ontario, London, ON, Canada N6A 5C1 Correspondence should be addressed to J. A. Kiernan, [email protected] Received 6 October 2011; Accepted 3 December 2011 Academic Editor: Seyed M. Mirsattari Copyright © 2012 J. A. Kiernan. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Only primates have temporal lobes, which are largest in man, accommodating 17% of the cerebral cortex and including areas with auditory, olfactory, vestibular, visual and linguistic functions. The hippocampal formation, on the medial side of the lobe, includes the parahippocampal gyrus, subiculum, hippocampus, dentate gyrus, and associated white matter, notably the fimbria, whose fibres continue into the fornix. The hippocampus is an inrolled gyrus that bulges into the temporal horn of the lateral ventricle. Association fibres connect all parts of the cerebral cortex with the parahippocampal gyrus and subiculum, which in turn project to the dentate gyrus. The largest efferent projection of the subiculum and hippocampus is through the fornix to the hypothalamus. The choroid fissure, alongside the fimbria, separates the temporal lobe from the optic tract, hypothalamus and midbrain. The amygdala comprises several nuclei on the medial aspect of the temporal lobe, mostly anterior the hippocampus and indenting the tip of the temporal horn. The amygdala receives input from the olfactory bulb and from association cortex for other modalities of sensation. -

Basic Brain Anatomy
Chapter 2 Basic Brain Anatomy Where this icon appears, visit The Brain http://go.jblearning.com/ManascoCWS to view the corresponding video. The average weight of an adult human brain is about 3 pounds. That is about the weight of a single small To understand how a part of the brain is disordered by cantaloupe or six grapefruits. If a human brain was damage or disease, speech-language pathologists must placed on a tray, it would look like a pretty unim- first know a few facts about the anatomy of the brain pressive mass of gray lumpy tissue (Luria, 1973). In in general and how a normal and healthy brain func- fact, for most of history the brain was thought to be tions. Readers can use the anatomy presented here as an utterly useless piece of flesh housed in the skull. a reference, review, and jumping off point to under- The Egyptians believed that the heart was the seat standing the consequences of damage to the structures of human intelligence, and as such, the brain was discussed. This chapter begins with the big picture promptly removed during mummification. In his and works down into the specifics of brain anatomy. essay On Sleep and Sleeplessness, Aristotle argued that the brain is a complex cooling mechanism for our bodies that works primarily to help cool and The Central Nervous condense water vapors rising in our bodies (Aristo- tle, republished 2011). He also established a strong System argument in this same essay for why infants should not drink wine. The basis for this argument was that The nervous system is divided into two major sec- infants already have Central nervous tions: the central nervous system and the peripheral too much moisture system The brain and nervous system. -

01 05 Lateral Surface of the Brain-NOTES.Pdf
Lateral Surface of the Brain Medical Neuroscience | Tutorial Notes Lateral Surface of the Brain 1 MAP TO NEUROSCIENCE CORE CONCEPTS NCC1. The brain is the body's most complex organ. LEARNING OBJECTIVES After study of the assigned learning materials, the student will: 1. Demonstrate the four paired lobes of the cerebral cortex and describe the boundaries of each. 2. Sketch the major features of each cerebral lobe, as seen from the lateral view, identifying major gyri and sulci that characterize each lobe. NARRATIVE by Leonard E. WHITE and Nell B. CANT Duke Institute for Brain Sciences Department of Neurobiology Duke University School of Medicine Overview When you view the lateral aspect of a human brain specimen (see Figures A3A and A102), three structures are usually visible: the cerebral hemispheres, the cerebellum, and part of the brainstem (although the brainstem is not visible in the specimen photographed in lateral view for Fig. 1 below). The spinal cord has usually been severed (but we’ll consider the spinal cord later), and the rest of the subdivisions are hidden from lateral view by the hemispheres. The diencephalon and the rest of the brainstem are visible on the medial surface of a brain that has been cut in the midsagittal plane. Parts of all of the subdivisions are also visible from the ventral surface of the whole brain. Over the next several tutorials, you will find video demonstrations (from the brain anatomy lab) and photographs (in the tutorial notes) of these brain surfaces, and sufficient detail in the narrative to appreciate the overall organization of the parts of the brain that are visible from each perspective. -

Journal of Neurotherapy: Investigations in Neuromodulation, Neurofeedback and Applied Neuroscience Technical Issues Involving Bipolar EEG Training Protocols John A
Journal of Neurotherapy: Investigations in Neuromodulation, Neurofeedback and Applied Neuroscience Technical Issues Involving Bipolar EEG Training Protocols John A. Putman MA and MS a a EEG Spectrum International , Encino, California E-mail: Published online: 08 Sep 2008. To cite this article: John A. Putman MA and MS (2001) Technical Issues Involving Bipolar EEG Training Protocols, Journal of Neurotherapy: Investigations in Neuromodulation, Neurofeedback and Applied Neuroscience, 5:3, 51-58 To link to this article: http://dx.doi.org/10.1300/J184v05n03_06 PLEASE SCROLL DOWN FOR ARTICLE © International Society for Neurofeedback and Research (ISNR), all rights reserved. This article (the “Article”) may be accessed online from ISNR at no charge. The Article may be viewed online, stored in electronic or physical form, or archived for research, teaching, and private study purposes. The Article may be archived in public libraries or university libraries at the direction of said public library or university library. Any other reproduction of the Article for redistribution, sale, resale, loan, sublicensing, systematic supply, or other distribution, including both physical and electronic reproduction for such purposes, is expressly forbidden. Preparing or reproducing derivative works of this article is expressly forbidden. ISNR makes no representation or warranty as to the accuracy or completeness of any content in the Article. From 1995 to 2013 the Journal of Neurotherapy was the official publication of ISNR (www. Isnr.org); on April 27, 2016 ISNR acquired the journal from Taylor & Francis Group, LLC. In 2014, ISNR established its official open-access journal NeuroRegulation (ISSN: 2373-0587; www.neuroregulation.org). THIS OPEN-ACCESS CONTENT MADE POSSIBLE BY THESE GENEROUS SPONSORS TECHNICAL NOTES The purpose of the Technical Notes section is to provide detailed technical descriptions and illustrations of software, hardware and tech- niques within our technically sophisticated field. -

1. Lateral View of Lobes in Left Hemisphere TOPOGRAPHY
TOPOGRAPHY T1 Division of Cerebral Cortex into Lobes 1. Lateral View of Lobes in Left Hemisphere 2. Medial View of Lobes in Right Hemisphere PARIETAL PARIETAL LIMBIC FRONTAL FRONTAL INSULAR: buried OCCIPITAL OCCIPITAL in lateral fissure TEMPORAL TEMPORAL 3. Dorsal View of Lobes 4. Ventral View of Lobes PARIETAL TEMPORAL LIMBIC FRONTAL OCCIPITAL FRONTAL OCCIPITAL Comment: The cerebral lobes are arbitrary divisions of the cerebrum, taking their names, for the most part, from overlying bones. They are not functional subdivisions of the brain, but serve as a reference for locating specific functions within them. The anterior (rostral) end of the frontal lobe is referred to as the frontal pole. Similarly, the anterior end of the temporal lobe is the temporal pole, and the posterior end of the occipital lobe the occipital pole. TOPOGRAPHY T2 central sulcus central sulcus parietal frontal occipital lateral temporal lateral sulcus sulcus SUMMARY CARTOON: LOBES SUMMARY CARTOON: GYRI Lateral View of Left Hemisphere central sulcus postcentral superior parietal superior precentral gyrus gyrus lobule frontal intraparietal sulcus gyrus inferior parietal lobule: supramarginal and angular gyri middle frontal parieto-occipital sulcus gyrus incision for close-up below OP T preoccipital O notch inferior frontal cerebellum gyrus: O-orbital lateral T-triangular sulcus superior, middle and inferior temporal gyri OP-opercular Lateral View of Insula central sulcus cut surface corresponding to incision in above figure insula superior temporal gyrus Comment: Insula (insular gyri) exposed by removal of overlying opercula (“lids” of frontal and parietal cortex). TOPOGRAPHY T3 Language sites and arcuate fasciculus. MRI reconstruction from a volunteer. central sulcus supramarginal site (posterior Wernicke’s) Language sites (squares) approximated from electrical stimulation sites in patients undergoing operations for epilepsy or tumor removal (Ojeman and Berger). -

Graduate Neuroanatomy GSBS GS141181
Page 1 Graduate Neuroanatomy GSBS GS141181 Laboratory Guide Offered and Coordinated by the Department of Neurobiology and Anatomy The University of Texas Health Science Center at Houston. This course guide was adatped from the Medical Neuroscience Laboratory Guide. Nachum Dafny, Ph.D., Course Director; Michael Beierlein, Ph.D., Laboratory Coordinator. Online teaching materials are available at https://oac22.hsc.uth.tmc.edu/courses/neuroanatomy/ Other course information available at http://openwetware.org/wiki/Beauchamp:GraduateNeuroanatomy Contents © 2000-Present University of Texas Health Science Center at Houston. All Rights Reserved. Unauthorized use of contents subject to civil and/or criminal prosecution. Graduate Neuroanatomy : Laboratory Guide Page 2 Table of Contents Overview of the Nervous System ................................................................................................................ 3 Laboratory Exercise #1: External Anatomy of the Brain ......................................................................... 19 Laboratory Exercise #2: Internal Organization of the Brain ..................................................................... 35 Graduate Neuroanatomy : Laboratory Guide Page 3 Overview of the Nervous System Nachum Dafny, Ph.D. The human nervous system is divided into the central nervous system (CNS) and the peripheral nervous system (PNS). The CNS, in turn, is divided into the brain and the spinal cord, which lie in the cranial cavity of the skull and the vertebral canal, respectively. The CNS and the PNS, acting in concert, integrate sensory information and control motor and cognitive functions. The Central Nervous System (CNS) The adult human brain weighs between 1200 to 1500g and contains about one trillion cells. It occupies a volume of about 1400cc - approximately 2% of the total body weight, and receives 20% of the blood, oxygen, and calories supplied to the body. The adult spinal cord is approximately 40 to 50cm long and occupies about 150cc. -

Human Anatomy and Physiology I Lab 10 the Nervous System
Human Anatomy and Physiology I Lab 10 The nervous system Learning Outcomes • Break the nervous system into its main subdivisions. Assessment: Exercise 10.1 • Visually locate and identify the major structures of the brain Assessment: Exercise 10.1 Overview of the nervous system Information The nervous system is the collection of cells and tissues that form the structures and organs involved in collecting and processing sensory information and then triggering reactions. The nervous system is broken down into the central nervous system and the peripheral nervous system. The central nervous system is just the brain and the spinal cord. The peripheral nervous system is the rest of the nervous tissue in the body and the sensory organs that the nervous tissue attaches to. The peripheral nervous tissue includes the cranial nerves that branch out from the brain and the spinal nerves that branch out from the spinal cord, as well as all the sensory organs in the head and body. Figure 10-1. A typical diagram of the nervous system, showing the central nervous system in yellow, and the nervous tissue of the peripheral nervous system in blue, but leaving out all the sensory organs that are also part of the peripheral nervous system. The four major regions of the brain Information As is the case with many other human organs, understanding the structures and anatomy of the brain has proven far easier than understanding its functioning and physiology. As a result, different neuroanatomists have organized and labelled the different parts of the brain in different ways, based on different criteria. -
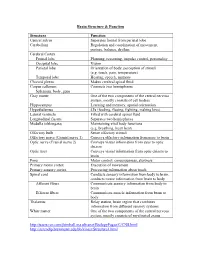
Brain Structure & Function Structure Function Central Sulcus Separates
Brain Structure & Function Structure Function Central sulcus Separates frontal from parietal lobe Cerebellum Regulation and coordination of movement, posture, balance, rhythm Cerebral Cortex Frontal lobe Planning, reasoning, impulse control, personality Occipital lobe Vision Parietal lobe Orientation of body, perception of stimuli (e.g. touch, pain, temperature) Temporal lobe Hearing, speech, memory Choroid plexus Makes cerebral spinal fluid Corpus callosum Connects two hemispheres Splenium, body, genu Gray matter One of the two components of the central nervous system, mostly consists of cell bodies Hippocampus Learning and memory, spatial orientation Hypothalamus 4Fs (feeding, fleeing, fighting, making love) Lateral ventricle Filled with cerebral spinal fluid Longitudinal fissure Separates two hemispheres Medulla (oblongata) Maintaining vital body functions (e.g. breathing, heart beat) Olfactory bulb Sense olfactory stimuli Olfactory nerve (Cranial nerve 1) Conveys olfactory information from nose to brain Optic nerve (Cranial nerve 2) Conveys visual information from eyes to optic chiasm Optic tract Conveys visual information from optic chiasm to brain Pons Motor control, consciousness, alertness Primary motor cortex Execution of movement Primary sensory cortex Processing information about touch Spinal cord Conducts sensory information from body to brain, conducts motor information from brain to body Afferent fibers Communicate sensory information from body to brain Efferent fibers Communicate muscle information from brain to body Thalamus Relay station, brain region that combines information from different sensory systems White matter One of the two components of the central nervous system, mostly consists of myelinated axons http://users.rcn.com/jkimball.ma.ultranet/BiologyPages/C/CNS.html http://serendip.brynmawr.edu/bb/kinser/Structure1.html . -

Anatomy of Cerebral Hemispheres Doctors Notes Notes/Extra Explanation Please View Our Editing File Before Studying This Lecture to Check for Any Changes
Color Code Important Anatomy of Cerebral Hemispheres Doctors Notes Notes/Extra explanation Please view our Editing File before studying this lecture to check for any changes. Objectives At the end of the lecture, the students should be able to: List the parts of the cerebral hemisphere (cortex, medulla, basal nuclei, lateral ventricle). Describe the subdivision of a cerebral hemisphere into lobes. List the important sulci and gyri of each lobe. Describe different types of fibers in cerebral medulla (association, projection and commissural) and give example of each type. Cerebrum Extra Corpus callosum o Largest part of the forebrain. ( makes up 2 / 3 rd weight off all brain) (recall: the forebrain gives the cerebral hemispheres and the diencephalon) o Divided into two halves, the cerebral hemispheres (right and left), which are separated Left hemisphere Right hemisphere by a deep median longitudinal fissure which lodges the falx cerebri*. o In the depth of the fissure, the hemispheres are connected by a bundle of fibers called the corpus callosum. *It is a large, crescent- shaped fold of meningeal layer of dura Median longitudinal fissure mater that descends vertically in the longitudinal fissure between the cerebral Extra Extra hemispheres Cerebrum Buried within the white matter Cerebral Hemispheres lie a number of nuclear masses The structure of cerebral hemipheres includes: (caudate, putamen, globus pallidus) collectively known as the basal ganglia. WM Deeper to the cortex, axons running to and from the cells of the cortex form an extensive mass of white matter (WM). Contains synapses (50 trillion) WM Superficial layer of grey matter, the cerebral cortex. -

Brain Anatomy
BRAIN ANATOMY Adapted from Human Anatomy & Physiology by Marieb and Hoehn (9th ed.) The anatomy of the brain is often discussed in terms of either the embryonic scheme or the medical scheme. The embryonic scheme focuses on developmental pathways and names regions based on embryonic origins. The medical scheme focuses on the layout of the adult brain and names regions based on location and functionality. For this laboratory, we will consider the brain in terms of the medical scheme (Figure 1): Figure 1: General anatomy of the human brain Marieb & Hoehn (Human Anatomy and Physiology, 9th ed.) – Figure 12.2 CEREBRUM: Divided into two hemispheres, the cerebrum is the largest region of the human brain – the two hemispheres together account for ~ 85% of total brain mass. The cerebrum forms the superior part of the brain, covering and obscuring the diencephalon and brain stem similar to the way a mushroom cap covers the top of its stalk. Elevated ridges of tissue, called gyri (singular: gyrus), separated by shallow groves called sulci (singular: sulcus) mark nearly the entire surface of the cerebral hemispheres. Deeper groves, called fissures, separate large regions of the brain. Much of the cerebrum is involved in the processing of somatic sensory and motor information as well as all conscious thoughts and intellectual functions. The outer cortex of the cerebrum is composed of gray matter – billions of neuron cell bodies and unmyelinated axons arranged in six discrete layers. Although only 2 – 4 mm thick, this region accounts for ~ 40% of total brain mass. The inner region is composed of white matter – tracts of myelinated axons. -

How Is the Brain Organized?
p CHAPTER 2 How Is the Brain Organized? An Overview of Brain Structure The Functional Organization Brain Terminology of the Brain The Brain’s Surface Features Principle 1: The Sequence of Brain Processing The Brain’s Internal Features Is “In Integrate Out” Microscopic Inspection: Cells and Fibers Principle 2: Sensory and Motor Divisions Exist Focus on Disorders: Meningitis and Throughout the Nervous System Encephalitis Principle 3: The Brain’s Circuits Are Crossed Focus on Disorders: Stroke Principle 4: The Brain Is Both Symmetrical and Asymmetrical Principle 5: The Nervous System Works A Closer Look at Neuroanatomy Through Excitation and Inhibition The Cranial Nervous System Principle 6: The Central Nervous System Has The Spinal Nervous System Multiple Levels of Function The Internal Nervous System Principle 7: Brain Systems Are Organized Both Focus on Disorders: Magendie, Bell, and Bell’s Hierarchically and in Parallel Palsy Principle 8: Functions in the Brain Are Both Localized and Distributed A. Klehr / Stone Images Micrograph: Carolina Biological Supply Co. / Phototake 36 I p hen buying a new car, people first inspect the In many ways, examining a brain for the first time is outside carefully, admiring the flawless finish similar to looking under the hood of a car. We have a vague W and perhaps even kicking the tires. Then they sense of what the brain does but no sense of how the parts open the hood and examine the engine, the part of the car that we see accomplish these tasks. We may not even be responsible for most of its behavior—and misbehavior. able to identify many of the parts. -

Dynamic Mapping of Human Cortical Development During Childhood Through Early Adulthood
Dynamic mapping of human cortical development during childhood through early adulthood Nitin Gogtay*†, Jay N. Giedd*, Leslie Lusk*, Kiralee M. Hayashi‡, Deanna Greenstein*, A. Catherine Vaituzis*, Tom F. Nugent III*, David H. Herman*, Liv S. Clasen*, Arthur W. Toga‡, Judith L. Rapoport*, and Paul M. Thompson‡ *Child Psychiatry Branch, National Institutes of Mental Health, National Institutes of Health, Bethesda, MD 20892; and ‡Laboratory of Neuro Imaging, Department of Neurology, University of California School of Medicine, Los Angeles, CA 90095-1769 Communicated by Leslie G. Ungerleider, National Institutes of Health, Bethesda, MD, April 15, 2004 (received for review January 7, 2004) We report the dynamic anatomical sequence of human cortical temporal cortex, which contains association areas that integrate gray matter development between the age of 4–21 years using information from several sensory modalities, matured last. Fur- quantitative four-dimensional maps and time-lapse sequences. thermore, the maturation of the cortex also appeared to follow Thirteen healthy children for whom anatomic brain MRI scans were the evolutionary sequence in which these regions were created. obtained every 2 years, for 8–10 years, were studied. By using models of the cortical surface and sulcal landmarks and a statistical Methods model for gray matter density, human cortical development could Subjects. Sample demographics are shown in Table 1. All subjects be visualized across the age range in a spatiotemporally detailed were recruited from the community for an ongoing National time-lapse sequence. The resulting time-lapse ‘‘movies’’ reveal that Institute of Mental Health study of human brain development (i) higher-order association cortices mature only after lower-order (20).