The Ecology and Conservation of Presbytis Rubicunda
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Monocyclic Phenolic Acids; Hydroxy- and Polyhydroxybenzoic Acids: Occurrence and Recent Bioactivity Studies
Molecules 2010, 15, 7985-8005; doi:10.3390/molecules15117985 OPEN ACCESS molecules ISSN 1420-3049 www.mdpi.com/journal/molecules Review Monocyclic Phenolic Acids; Hydroxy- and Polyhydroxybenzoic Acids: Occurrence and Recent Bioactivity Studies Shahriar Khadem * and Robin J. Marles Natural Health Products Directorate, Health Products and Food Branch, Health Canada, 2936 Baseline Road, Ottawa, Ontario K1A 0K9, Canada * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +1-613-954-7526; Fax: +1-613-954-1617. Received: 19 October 2010; in revised form: 3 November 2010 / Accepted: 4 November 2010 / Published: 8 November 2010 Abstract: Among the wide diversity of naturally occurring phenolic acids, at least 30 hydroxy- and polyhydroxybenzoic acids have been reported in the last 10 years to have biological activities. The chemical structures, natural occurrence throughout the plant, algal, bacterial, fungal and animal kingdoms, and recently described bioactivities of these phenolic and polyphenolic acids are reviewed to illustrate their wide distribution, biological and ecological importance, and potential as new leads for the development of pharmaceutical and agricultural products to improve human health and nutrition. Keywords: polyphenols; phenolic acids; hydroxybenzoic acids; natural occurrence; bioactivities 1. Introduction Phenolic compounds exist in most plant tissues as secondary metabolites, i.e. they are not essential for growth, development or reproduction but may play roles as antioxidants and in interactions between the plant and its biological environment. Phenolics are also important components of the human diet due to their potential antioxidant activity [1], their capacity to diminish oxidative stress- induced tissue damage resulted from chronic diseases [2], and their potentially important properties such as anticancer activities [3-5]. -

Primates of the Southern Mentawai Islands
Primate Conservation 2018 (32): 193-203 The Status of Primates in the Southern Mentawai Islands, Indonesia Ahmad Yanuar1 and Jatna Supriatna2 1Department of Biology and Post-graduate Program in Biology Conservation, Tropical Biodiversity Conservation Center- Universitas Nasional, Jl. RM. Harsono, Jakarta, Indonesia 2Department of Biology, FMIPA and Research Center for Climate Change, University of Indonesia, Depok, Indonesia Abstract: Populations of the primates native to the Mentawai Islands—Kloss’ gibbon Hylobates klossii, the Mentawai langur Presbytis potenziani, the Mentawai pig-tailed macaque Macaca pagensis, and the snub-nosed pig-tailed monkey Simias con- color—persist in disturbed and undisturbed forests and forest patches in Sipora, North Pagai and South Pagai. We used the line-transect method to survey primates in Sipora and the Pagai Islands and estimate their population densities. We walked 157.5 km and 185.6 km of line transects on Sipora and on the Pagai Islands, respectively, and obtained 93 sightings on Sipora and 109 sightings on the Pagai Islands. On Sipora, we estimated population densities for H. klossii, P. potenziani, and S. concolor in an area of 9.5 km², and M. pagensis in an area of 12.6 km². On the Pagai Islands, we estimated the population densities of the four primates in an area of 11.1 km². Simias concolor was found to have the lowest group densities on Sipora, whilst P. potenziani had the highest group densities. On the Pagai Islands, H. klossii was the least abundant and M. pagensis had the highest group densities. Primate populations, notably of the snub-nosed pig-tailed monkey and Kloss’ gibbon, are reduced and threatened on the southern Mentawai Islands. -

Habitat Utilization and Feeding Biology of Himalayan Grey Langur
动 物 学 研 究 2010,Apr. 31(2):177−188 CN 53-1040/Q ISSN 0254-5853 Zoological Research DOI:10.3724/SP.J.1141.2010.02177 Habitat Utilization and Feeding Biology of Himalayan Grey Langur (Semnopithecus entellus ajex) in Machiara National Park, Azad Jammu and Kashmir, Pakistan Riaz Aziz Minhas1,*, Khawaja Basharat Ahmed2, Muhammad Siddique Awan2, Naeem Iftikhar Dar3 (1. World Wide Fund for Nature-Pakistan (WWF-Pakistan) AJK Office Muzaffarabad, Azad Jammu and Kashmir 13100, Pakistan; 2. Department ofZoology, University of Azad Jammu and Kashmir Muzaffarabad, Azad Jammu and Kashmir 13100, Pakistan; 3. Department of Wildlife and Fisheries, Government of Azad Jammu and Kashmir, Muzaffarabad, Azad Jammu and Kashmir 13100, Pakistan) Abstract: Habitat utilization and feeding biology of Himalayan Grey Langur (Semnopithecus entellus ajex) were studied from April, 2006 to April, 2007 in Machiara National Park, Azad Jammu and Kashmir, Pakistan. The results showed that in the winter season the most preferred habitat of the langurs was the moist temperate coniferous forests interspersed with deciduous trees, while in the summer season they preferred to migrate into the subalpine scrub forests at higher altitudes. Langurs were folivorous in feeding habit, recorded as consuming more than 49 plant species (27 in summer and 22 in winter) in the study area. The mature leaves (36.12%) were preferred over the young leaves (27.27%) while other food components comprised of fruits (17.00%), roots (9.45%), barks (6.69%), flowers (2.19%) and stems (1.28%) of various plant species. Key words: Himalayan Grey Langur; Habitat; Food biology; Machiara National Park 喜马拉雅灰叶猴栖息地利用和食性生物学 Riaz Aziz Minhas1,*, Khawaja Basharat Ahmed2, Muhammad Siddique Awan2, Naeem Iftikhar Dar3 (1. -

Nonhuman Primates
Zoological Studies 42(1): 93-105 (2003) Dental Variation among Asian Colobines (Nonhuman Primates): Phylogenetic Similarities or Functional Correspondence? Ruliang Pan1,2,* and Charles Oxnard1 1School of Anatomy and Human Biology, University of Western Australia, Crawley, Perth, WA 6009, Australia 2Institute of Zoology, Chinese Academy of Sciences, Beijing 100080, China (Accepted August 27, 2002) Ruliang Pan and Charles Oxnard (2003) Dental variation among Asian colobines (nonhuman primates): phy- logenetic similarities or functional correspondence? Zoological Studies 42(1): 93-105. In order to reveal varia- tions among Asian colobines and to test whether the resemblance in dental structure among them is mainly associated with similarities in phylogeny or functional adaptation, teeth of 184 specimens from 15 Asian colobine species were measured and studied by performing bivariate (allometry) and multivariate (principal components) analyses. Results indicate that each tooth shows a significant close relationship with body size. Low negative and positive allometric scales for incisors and molars (M2s and M3s), respectively, are each con- sidered to be related to special dental modifications for folivorous preference of colobines. Sexual dimorphism in canine eruption reported by Harvati (2000) is further considered to be associated with differences in growth trajectories (allometric pattern) between the 2 sexes. The relationships among the 6 genera of Asian colobines found greatly differ from those proposed in other studies. Four groups were detected: 1) Rhinopithecus, 2) Semnopithecus, 3) Trachypithecus, and 4) Nasalis, Pygathrix, and Presbytis. These separations were mainly determined by differences in molar structure. Molar sizes of the former 2 groups are larger than those of the latter 2 groups. -

Title SOS : Save Our Swamps for Peat's Sake Author(S) Hon, Jason Citation
Title SOS : save our swamps for peat's sake Author(s) Hon, Jason SANSAI : An Environmental Journal for the Global Citation Community (2011), 5: 51-65 Issue Date 2011-04-12 URL http://hdl.handle.net/2433/143608 Right Type Journal Article Textversion publisher Kyoto University SOS: save our swamps for peatʼs sake JASON HON Abstract The Malaysian governmentʼs scheme for the agricultural intensification of oil palm production is putting increasing pressure on lowland areas dominated by peat swamp forests.This paper focuses on the peat swamp forests of Sarawak, home to 64 per cent of the peat swamp forests in Malaysia and earmarked under the Malaysian governmentʼs Third National Agriculture Policy (1998-2010) for the development and intensification of the oil palm industry.Sarawakʼs tropical peat swamp forests form a unique ecosystem, where rare plant and animal species, such as the alan tree and the red-banded langur, can be found.They also play a vital role in maintaining the carbon balance, storing up to 10 times more carbon per hectare than other tropical forests. Draining these forests for agricultural purposes endangers the unique species of flora and fauna that live in them and increases the likelihood of uncontrollable peat fires, which emit lethal smoke that can pose a huge environmental risk to the health of humans and wildlife.This paper calls for a radical reassessment of current agricultural policies by the Malaysian government and highlights the need for concerted effort to protect the fragile ecosystems of Sarawakʼs endangered -

Deforestation of Primate Habitat on Sumatra and Adjacent Islands, Indonesia
Primate Conservation 2017 (31): 71-82 Deforestation of Primate Habitat on Sumatra and Adjacent Islands, Indonesia Jatna Supriatna1,2, Asri A. Dwiyahreni2, Nurul Winarni2, Sri Mariati3,4 and Chris Margules2,5 ¹Department of Biology, FMIPA Universitas Indonesia, Depok, Indonesia 2Research Center for Climate Change, Universitas Indonesia, Depok, Indonesia 3Postgraduate Program, Trisakti Institute for Tourism, Pesanggrahan, Jakarta, Indonesia 4Conservation International, Indonesia 5Centre for Tropical Environmental and Sustainability Science, College of Marine and Environmental Sciences, James Cook University, Cairns, Australia Abstract: The severe declines in forest cover on Sumatra and adjacent islands have been well-documented but that has not slowed the rate of forest loss. Here we present recent data on deforestation rates and primate distribution patterns to argue, yet again, for action to avert potential extinctions of Sumatran primates in the near future. Maps of forest loss were constructed using GIS and satellite imagery. Maps of primate distributions were estimated from published studies, museum records and expert opinion, and the two were overlaid on one another. The extent of deforestation in the provinces of Sumatra between 2000 and 2012 varied from 3.74% (11,599.9 ha in Lampung) to 49.85% (1,844,804.3 ha in Riau), with the highest rates occurring in the provinces of Riau, Jambi, Bangka Belitung and South Sumatra. During that time six species lost 50% or more of their forest habitat: the Banded langur Presbytis femoralis lost 82%, the Black-and-white langur Presbytis bicolor lost 78%, the Black-crested Sumatran langur Presbytis melalophos and the Bangka slow loris Nycticebus bancanus both lost 62%, the Lar gibbon Hylobates lar lost 54%, and the Pale-thighed langur Presbytis siamensis lost 50%. -

The Role of Exposure in Conservation
Behavioral Application in Wildlife Photography: Developing a Foundation in Ecological and Behavioral Characteristics of the Zanzibar Red Colobus Monkey (Procolobus kirkii) as it Applies to the Development Exhibition Photography Matthew Jorgensen April 29, 2009 SIT: Zanzibar – Coastal Ecology and Natural Resource Management Spring 2009 Advisor: Kim Howell – UDSM Academic Director: Helen Peeks Table of Contents Acknowledgements – 3 Abstract – 4 Introduction – 4-15 • 4 - The Role of Exposure in Conservation • 5 - The Zanzibar Red Colobus (Piliocolobus kirkii) as a Conservation Symbol • 6 - Colobine Physiology and Natural History • 8 - Colobine Behavior • 8 - Physical Display (Visual Communication) • 11 - Vocal Communication • 13 - Olfactory and Tactile Communication • 14 - The Importance of Behavioral Knowledge Study Area - 15 Methodology - 15 Results - 16 Discussion – 17-30 • 17 - Success of the Exhibition • 18 - Individual Image Assessment • 28 - Final Exhibition Assessment • 29 - Behavioral Foundation and Photography Conclusion - 30 Evaluation - 31 Bibliography - 32 Appendices - 33 2 To all those who helped me along the way, I am forever in your debt. To Helen Peeks and Said Hamad Omar for a semester of advice, and for trying to make my dreams possible (despite the insurmountable odds). Ali Ali Mwinyi, for making my planning at Jozani as simple as possible, I thank you. I would like to thank Bi Ashura, for getting me settled at Jozani and ensuring my comfort during studies. Finally, I am thankful to the rangers and staff of Jozani for welcoming me into the park, for their encouragement and support of my project. To Kim Howell, for agreeing to support a project outside his area of expertise, I am eternally grateful. -
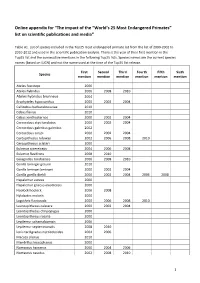
Online Appendix for “The Impact of the “World's 25 Most Endangered
Online appendix for “The impact of the “World’s 25 Most Endangered Primates” list on scientific publications and media” Table A1. List of species included in the Top25 most endangered primate list from the list of 2000-2002 to 2010-2012 and used in the scientific publication analysis. There is the year of their first mention in the Top25 list and the consecutive mentions in the following Top25 lists. Species names are the current species names (based on IUCN) and not the name used at the time of the Top25 list release. First Second Third Fourth Fifth Sixth Species mention mention mention mention mention mention Ateles fusciceps 2006 Ateles hybridus 2006 2008 2010 Ateles hybridus brunneus 2004 Brachyteles hypoxanthus 2000 2002 2004 Callicebus barbarabrownae 2010 Cebus flavius 2010 Cebus xanthosternos 2000 2002 2004 Cercocebus atys lunulatus 2000 2002 2004 Cercocebus galeritus galeritus 2002 Cercocebus sanjei 2000 2002 2004 Cercopithecus roloway 2002 2006 2008 2010 Cercopithecus sclateri 2000 Eulemur cinereiceps 2004 2006 2008 Eulemur flavifrons 2008 2010 Galagoides rondoensis 2006 2008 2010 Gorilla beringei graueri 2010 Gorilla beringei beringei 2000 2002 2004 Gorilla gorilla diehli 2000 2002 2004 2006 2008 Hapalemur aureus 2000 Hapalemur griseus alaotrensis 2000 Hoolock hoolock 2006 2008 Hylobates moloch 2000 Lagothrix flavicauda 2000 2006 2008 2010 Leontopithecus caissara 2000 2002 2004 Leontopithecus chrysopygus 2000 Leontopithecus rosalia 2000 Lepilemur sahamalazensis 2006 Lepilemur septentrionalis 2008 2010 Loris tardigradus nycticeboides -

REVIEW ARTICLE Agroecosystems and Primate Conservation in the Tropics: a Review
American Journal of Primatology 74:696–711 (2012) REVIEW ARTICLE Agroecosystems and Primate Conservation in The Tropics: A Review ∗ ALEJANDRO ESTRADA1 , BECKY E. RABOY2,3, AND LEONARDO C. OLIVEIRA3-6 1Estaci´on de Biolog´ıa Tropical Los Tuxtlas Instituto de Biolog´ıa, Universidad Nacional Aut´onoma de M´exico, Mexico City, Mexico 2Conservation Ecology Center, Smithsonian Conservation Biology Institute, National Zoological Park, Washington, DC 3Instituto de Estudos S´ocioambientais do Sul da Bahia (IESB), Ilh´eus-BA, Brazil 4Programa de P´os-Gradua¸c˜ao em Ecologia, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil 5Programa de P´os-Gradua¸c˜ao em Ecologia e Conserva¸c˜ao da Biodiversidade, Universidade Estadual de Santa Cruz, Ilh´eus-BA, Brazil 6Bicho do Mato Instituto de Pesquisa, Belo Horizonte-MG, Brazil Agroecosystems cover more than one quarter of the global land area (ca. 50 million km2) as highly simplified (e.g. pasturelands) or more complex systems (e.g. polycultures and agroforestry systems) with the capacity to support higher biodiversity. Increasingly more information has been published about primates in agroecosystems but a general synthesis of the diversity of agroecosystems that primates use or which primate taxa are able to persist in these anthropogenic components of the landscapes is still lacking. Because of the continued extensive transformation of primate habitat into human-modified landscapes, it is important to explore the extent to which agroecosystems are used by primates. In this article, we reviewed published information on the use of agroecosystems by primates in habitat countries and also discuss the potential costs and benefits to human and nonhuman primates of primate use of agroecosystems. -

Chemical Investigation of the Bark of Adenanthera Pavonina Linn Arzumand Ara, Md
Int. J. Chem. Sci.: 10(1), 2012, 98-103 ISSN 0972-768X www.sadgurupublications.com CHEMICAL INVESTIGATION OF THE BARK OF ADENANTHERA PAVONINA LINN ARZUMAND ARA, MD. ABUL HASHEM* and TANVIR MUSLIMa Department of Chemistry, Jahangirnagar University, Savar, DHAKA – 1342, BANGLADESH aDepartment of Chemistry, University of Dhaka, DHAKA – 1000, BANGLADESH ABSTRACT The chemical investigation of the bark of Adenanthera pavonina Linn. have been found to contain the reducing sugar (1.01%) as glucose. The percentages of various amino acids present in the crude protein (5.25%) were found to be aspartic acid (0.10%), threonine (0.24%), serine (0.08%), glutamic acid (0.52%), glycine (0.09%), alanine (0.07%), valine (0.10%), methionine (0.13%), isoleucine (0.06%), tyrosine (0.27%), histidine (0.11%), lysine (0.88%) and arginine (0.25%). The fatty acid composition were found to be lauric (5.23%), palmitic (38.16%), oleic acid (6.29%) and stearic acid (8.93%). Key words: Adenanthera pavonina Linn, Reducing sugar, Amino acids, Fatty Acids. INTRODUCTION The plant Adenanthera pavonina Linn. (Bengali Rakta Kambal) belongs to the family Leguminosae and is an important medicinal plant widely distributed in the Asian and African countries1. As an indigenous plant it is grown and cultivated mostly in the south- eastern region of Bangladesh2. Decoction of the seeds is used in pulmonary affections and externally applied in chronic ophthalmia. The seeds of the plant are used for the treatment of boils and inflammations2,3. Methanolic extract of the seeds and roots showed blood pressure lowering effect2,4. The leaves and the barks of the plant are used as a remedy for chronic rheumatism, gout, haematuria, haematemesis and diarrhoea1-3. -

Colobus Angolensis Palliatus) in an East African Coastal Forest
African Primates 7 (2): 203-210 (2012) Activity Budgets of Peters’ Angola Black-and-White Colobus (Colobus angolensis palliatus) in an East African Coastal Forest Zeno Wijtten1, Emma Hankinson1, Timothy Pellissier2, Matthew Nuttall1 & Richard Lemarkat3 1Global Vision International (GVI) Kenya 2University of Winnipeg, Winnipeg, Canada 3Kenyan Wildlife Services (KWS), Kenya Abstract: Activity budgets of primates are commonly associated with strategies of energy conservation and are affected by a range of variables. In order to establish a solid basis for studies of colobine monkey food preference, food availability, group size, competition and movement, and also to aid conservation efforts, we studied activity of individuals from social groups of Colobus angolensis palliatus in a coastal forest patch in southeastern Kenya. Our observations (N = 461 hours) were conducted year-round, over a period of three years. In our Colobus angolensis palliatus study population, there was a relatively low mean group size of 5.6 ± SD 2.7. Resting, feeding, moving and socializing took up 64%, 22%, 3% and 4% of their time, respectively. In the dry season, as opposed to the wet season, the colobus increased the time they spent feeding, traveling, and being alert, and decreased the time they spent resting. General activity levels and group sizes are low compared to those for other populations of Colobus spp. We suggest that Peters’ Angola black-and-white colobus often live (including at our study site) under low preferred-food availability conditions and, as a result, are adapted to lower activity levels. With East African coastal forest declining rapidly, comparative studies focusing on C. -

A Note on Responses of Juvenile Javan Lutungs (Trachypithecus Auratus Mauritius) Against Attempted Predation by Crested Goshawks (Accipter Trivirgatus)
人と自然 Humans and Nature 25: 105−110 (2014) Report A note on responses of juvenile Javan lutungs (Trachypithecus auratus mauritius) against attempted predation by crested goshawks (Accipter trivirgatus) Yamato TSUJI 1*, Hiroyoshi HIGUCHI 2 and Bambang SURYOBROTO 3 1 Primate Research Institute, Kyoto University, Inuyama, Japan 2 Graduate School of Media and Governance, Keio University, Fujisawa, Japan 3 Bogor Agricultural University, Java West, Indonesia Abstract On October 31st, 2013, we unexpectedly observed attempted predation of two juvenile Javan lutungs (Trachypithecus auratus mauritius) by adult crested goshawks (Accipter trivirgatus), in Pangandaran Nature Reserve, West Java, Indonesia. The goshawks flew over a group of feeding lutungs, attempting attacks only on juvenile lutungs from the rear, while emitting tweeting calls. The attacks involved two different birds (probably a pair) in turn from different directions. The attacks by the goshawks were repeated six times during our observation period (between 13:51 and 13:58), but none of the attempts was successful. During the attacks, no other lutungs in the group showed any anti-predator behavior (such as emitting alarm calls or escaping) against the goshawks perhaps because their body weight (6-10 kg) was much larger than that of goshawk (0.4-0.6 kg). To our knowledge, this is the first detailed report of hunting-related behavior to primates by this raptor species. Key words: Accipter trivirgatus, crested goshawk, Javan lutung, Pangandaran, predation, Trachypithecus auratus mauritius Introduction primates has been conducted for over 50 years. However, with the exception of Philippine eagles Predation is considered to be a principal selective (Pithecophaga jefferyi) and hawk-eagles (Spizaetus force leading to the evolution of behavioral traits spp.), reports on the predation of primates by raptors and social systems in primates (van Schaik, 1983; in Asia are limited (Ferguson-Lee and Christie, 2001; Hart, 2007), although there are few case studies on Hart, 2007; Fam and Nijman, 2011).