Chemical Composition and Fermentation Studies of Citron '
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bright Citron™ Soak
Product Profile BRIGHT CITRON™ SOAK WHAT IT IS An aromatic and refreshing sea salt soak for cleansing skin. WHAT IT DOES Softens and cleanses skin. WHY YOU NEED IT • Provides the first step to cleanse impurities and soften skin for a luxurious manicure or pedicure experience. • Gently cleanses without drying skin. FRAGRANCE FEATURED Pink Grapefruit and Warm Amber. FRAGRANCE PROFILE • Bright citrus top notes. • Soft floral mid notes. CREATIVE SUGGESTIONS • Light woody base notes. • Create a fragrant ceremonial experience by placing the Soak into warm water directly prior to ACTIVE BOTANICALS immersing hands or feet. Kaffir Lime (Citrus Hystrix Leaf Extract): • Add lime or grapefruit slices to enhance the Is known to purify skin and promote radiance. experience. • Use as a foot soak prior to all salon services. Honey: • Excellent retail product for home use in bath tubs. Is known to hydrate skin and help retain moisture. INGREDIENT LISTING FEATURED INGREDIENTS & BENEFITS Maris Sal ((Sea Salt) Sel Marin), Sodium Sesquicarbonate, Sea Salt: Argania Spinosa Kernel Oil, Honey, Citrus Hystrix Leaf Natural salt used for its therapeutic properties. Extract, Aqua ((Water) Eau), Glycerin, Propylene Glycol, Salts increase the flow of water in and out of Hydrated Silica, Parfum (Fragrance), Limonene, Linalool, cells, in essence flushing and cleansing the cells. Hexyl Cinnamal, Citral. This facilitates the absorption of other ingredients into the cells. AVAILABLE SIZES • Retail/Professional: 410 g (14.4 oz) Argan Oil (Argania Spinosa Kernel Oil): • Professional: 3.3 kg (118.8 oz) Is known to nourish dry skin. DIRECTIONS FOR USE • For feet, add a teaspoon to footbath and agitate water to dissolve. -

Cinnamon Kumquats
Preserve Today, Relish Tomorrow UCCE Master Food Preservers of El Dorado County 311 Fair Lane, Placerville CA 95667 Helpline (530) 621-5506 • Email: [email protected] • Visit us on Facebook and Twitter! Cinnamon Kumquats “How about a kumquat, my little chickadee?” (W.C. Fields, My Little Chickadee,1940) Say what? Yes, I said kumquats. Those adorable little kumquats. You know, those “things” that you have been so curious about. Another idea for using citrus that is not a marmalade. Vive la différence! That said, a kumquat marmalade is nothing short of marvelous. Honestly. “A kumquat is not an orange though it wants to be one, especially when they’re around other kumquats. (W.C. Fields, It’s A Gift, 1934) Kumquats are native to China, and their name comes from the Cantonese kam kwat, which means "golden orange." They are a symbol of prosperity and a traditional gift at Lunar New Year. Unlike other citrus, kumquats are eaten whole, including the skin. They have a tart-bitter-sweet taste that is boldly refreshing. Ya gotta try one. Really. Just pop one in your mouth and go for it. Fresh kumquats are wonderful in salads and in savory dishes. They are also great in chutneys and relishes. We canned them in a sweet cinnamon syrup. They can then be eaten right out of the jar like candy or used in desserts such as pound cakes or cheesecakes. The syrup is wonderful for drizzles, too. Savory ideas: use them in salads (use the syrup in your dressing!), they would be perfect with ham, maybe as a glaze for chicken wings (I would add some hot sauce, too). -

Tropical Horticulture: Lecture 32 1
Tropical Horticulture: Lecture 32 Lecture 32 Citrus Citrus: Citrus spp., Rutaceae Citrus are subtropical, evergreen plants originating in southeast Asia and the Malay archipelago but the precise origins are obscure. There are about 1600 species in the subfamily Aurantioideae. The tribe Citreae has 13 genera, most of which are graft and cross compatible with the genus Citrus. There are some tropical species (pomelo). All Citrus combined are the most important fruit crop next to grape. 1 Tropical Horticulture: Lecture 32 The common features are a superior ovary on a raised disc, transparent (pellucid) dots on leaves, and the presence of aromatic oils in leaves and fruits. Citrus has increased in importance in the United States with the development of frozen concentrate which is much superior to canned citrus juice. Per-capita consumption in the US is extremely high. Citrus mitis (calamondin), a miniature orange, is widely grown as an ornamental house pot plant. History Citrus is first mentioned in Chinese literature in 2200 BCE. First citrus in Europe seems to have been the citron, a fruit which has religious significance in Jewish festivals. Mentioned in 310 BCE by Theophrastus. Lemons and limes and sour orange may have been mutations of the citron. The Romans grew sour orange and lemons in 50–100 CE; the first mention of sweet orange in Europe was made in 1400. Columbus brought citrus on his second voyage in 1493 and the first plantation started in Haiti. In 1565 the first citrus was brought to the US in Saint Augustine. 2 Tropical Horticulture: Lecture 32 Taxonomy Citrus classification based on morphology of mature fruit (e.g. -

Facts About Citrus Fruits and Juices: Grapefruit1 Gail C
Archival copy: for current recommendations see http://edis.ifas.ufl.edu or your local extension office. FSHN02-6 Facts About Citrus Fruits and Juices: Grapefruit1 Gail C. Rampersaud2 Grapefruit is a medium- to large-sized citrus fruit. It is larger than most oranges and the fruit may be flattened at both ends. The skin is mostly yellow but may include shades of green, white, or pink. Skin color is not a sign of ripeness. Grapefruit are fully ripe when picked. Popular varieties of Florida grapefruit include: Did you know… Marsh White - white to amber colored flesh and almost seedless. Grapefruit was first Ruby Red - pink to reddish colored flesh with few seeds. discovered in the West Flame - red flesh and mostly seedless. Indies and introduced to Florida in the 1820s. Most grapefruit in the U.S. is still grown in Florida. Compared to most citrus fruits, grapefruit have an extended growing season and several Florida Grapefruit got its name because it grows in varieties grow from September through June. clusters on the tree, just like grapes! Fresh citrus can be stored in any cool, dry place but will last longer if stored in the refrigerator. Do Imposter!! not store fresh grapefruit in plastic bags or film- wrapped trays since this may cause mold to grow on the fruit. Whether you choose white or pink grapefruit or grapefruit juice, you’ll get great taste and a variety of health benefits! Read on…. 1. This document is FSHN026, one of a series of the Food Science and Human Nutrition Department, Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences, University of Florida. -
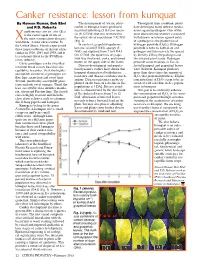
Canker Resistance: Lesson from Kumquat by Naveen Kumar, Bob Ebel the Development of Asiatic Citrus Throughout Their Evolution, Plants and P.D
Canker resistance: lesson from kumquat By Naveen Kumar, Bob Ebel The development of Asiatic citrus Throughout their evolution, plants and P.D. Roberts canker in kumquat leaves produced have developed many defense mecha- anthomonas citri pv. citri (Xcc) localized yellowing (5 DAI) or necro- nisms against pathogens. One of the is the causal agent of one of sis (9-12 DAI) that was restricted to most characteristic features associated the most serious citrus diseases the actual site of inoculation 7-12 DAI with disease resistance against entry X (Fig. 2). of a pathogen is the production of worldwide, Asiatic citrus canker. In the United States, Florida experienced In contrast, grapefruit epidermis hydrogen peroxide (H2O2). Hydrogen three major outbreaks of Asiatic citrus became raised (5 DAI), spongy (5 peroxide is toxic to both plant and canker in 1910, 1984 and 1995, and it DAI) and ruptured from 7 to 8 DAI. pathogen and thus restricts the spread is a constant threat to the $9 billion On 12 DAI, the epidermis of grape- by directly killing the pathogen and citrus industry. fruit was thickened, corky, and turned the infected plant tissue. Hydrogen Citrus genotypes can be classified brown on the upper side of the leaves. peroxide concentrations in Xcc-in- into four broad classes based on sus- Disease development and popula- fected kumquat and grapefruit leaves ceptibility to canker. First, the highly- tion dynamics studies have shown that were different. Kumquat produces susceptible commercial genotypes are kumquat demonstrated both disease more than three times the amount of Key lime, grapefruit and sweet lime. -

The Asian Citrus Psyllid and the Citrus Disease Huanglongbing
TheThe AsianAsian CitrusCitrus PsyllidPsyllid andand thethe CitrusCitrus DiseaseDisease HuanglongbingHuanglongbing Psyllid Huanglongbing The psyllid (pronounced síl - lid) is a small insect, about the size of an aphid The pest insect It has an egg stage, 5 wingless intermediate stages called nymphs, and winged adults Adult The pest insect Egg 5 Nymphs (insects molt to grow bigger) Adult psyllids usually feed on the underside of leaves and can feed on either young or mature leaves. This allows adults to survive year -round. The pest insect When feeding, the adult leans forward on its elbows and tips its rear end up in a very characteristic 45 o angle. The eggs are yellow -orange, tucked into the tips of tiny new leaves, and they are difficult to see because they are so small The pest insect The nymphs produce waxy tubules that direct the honeydew away from their bodies. These waxy tubules are unique and easy to recognize. Nymphs can only survive by living on young, tender The leaves and stems. pest insect Thus, nymphs are found only when the plant is producing new leaves. As Asian citrus psyllid feeds, it injects a salivary toxin that causes the tips of new leaves to easily break off. If the leaf survives, then it twists as it grows. Twisted leaves can be a sign that the psyllid has been there. The pest insect What plants can the psyllid attack? All types of citrus and closely related plants in the Rutaceae family • Citrus (limes, lemons, oranges, grapefruit, mandarins…) • Fortunella (kumquats) • Citropsis (cherry orange) • Murraya paniculata (orange jasmine) • Bergera koenigii (Indian curry leaf) • Severinia buxifolia (Chinese box orange) Plants • Triphasia trifolia (limeberry) • Clausena indica (wampei) affected • Microcitrus papuana (desert-lime) • Others…. -

Citrus from Seed?
Which citrus fruits will come true to type Orogrande, Tomatera, Fina, Nour, Hernandina, Clementard.) from seed? Ellendale Tom McClendon writes in Hardy Citrus Encore for the South East: Fortune Fremont (50% monoembryonic) “Most common citrus such as oranges, Temple grapefruit, lemons and most mandarins Ugli Umatilla are polyembryonic and will come true to Wilking type. Because most citrus have this trait, Highly polyembryonic citrus types : will mostly hybridization can be very difficult to produce nucellar polyembryonic seeds that will grow true to type. achieve…. This unique characteristic Citrus × aurantiifolia Mexican lime (Key lime, West allows amateurs to grow citrus from seed, Indian lime) something you can’t do with, say, Citrus × insitorum (×Citroncirus webberii) Citranges, such as Rusk, Troyer etc. apples.” [12*] Citrus × jambhiri ‘Rough lemon’, ‘Rangpur’ lime, ‘Otaheite’ lime Monoembryonic (don’t come true) Citrus × limettioides Palestine lime (Indian sweet lime) Citrus × microcarpa ‘Calamondin’ Meyer Lemon Citrus × paradisi Grapefruit (Marsh, Star Ruby, Nagami Kumquat Redblush, Chironja, Smooth Flat Seville) Marumi Kumquat Citrus × sinensis Sweet oranges (Blonde, navel and Pummelos blood oranges) Temple Tangor Citrus amblycarpa 'Nasnaran' mandarin Clementine Mandarin Citrus depressa ‘Shekwasha’ mandarin Citrus karna ‘Karna’, ‘Khatta’ Poncirus Trifoliata Citrus kinokuni ‘Kishu mandarin’ Citrus lycopersicaeformis ‘Kokni’ or ‘Monkey mandarin’ Polyembryonic (come true) Citrus macrophylla ‘Alemow’ Most Oranges Citrus reshni ‘Cleopatra’ mandarin Changshou Kumquat Citrus sunki (Citrus reticulata var. austera) Sour mandarin Meiwa Kumquat (mostly polyembryonic) Citrus trifoliata (Poncirus trifoliata) Trifoliate orange Most Satsumas and Tangerines The following mandarin varieties are polyembryonic: Most Lemons Dancy Most Limes Emperor Grapefruits Empress Tangelos Fairchild Kinnow Highly monoembryonic citrus types: Mediterranean (Avana, Tardivo di Ciaculli) Will produce zygotic monoembryonic seeds that will not Naartje come true to type. -

Citrus Fruits 2020 Summary (August 2020) 3 USDA, National Agricultural Statistics Service
United States Department of Citrus Fruits Agriculture National 2020 Summary Agricultural Statistics Service August 2020 ISSN: 1948-9048 Contents Utilized Citrus Production – United States Chart ................................................................................................................... 6 Citrus Value of Production – United States Chart .................................................................................................................. 6 Citrus Narrative ....................................................................................................................................................................... 7 Citrus Acreage, Production, Utilization, and Value – States and United States: 2017-2018, 2018-2019, and 2019-2020 ........................................................................................................................................................................ 8 Citrus Acreage, Production, Utilization, and Value by Crop – United States: 2017-2018, 2018-2019, and 2019-2020 ........................................................................................................................................................................ 9 Orange Acreage, Yield, Utilization, Price, and Value by Type – States and United States: 2017-2018, 2018-2019, and 2019-2020 ................................................................................................................................................... 10 Bearing Acres of Oranges – United States Chart ................................................................................................................. -

Citron Watermelon Potential to Improve Crop Diversification and Reduce Negative Impacts of Climate Change
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 12 January 2021 doi:10.20944/preprints202101.0213.v1 Review Citron watermelon potential to improve crop diversification and reduce negative impacts of climate change Takudzwa Mandizvo 1*, Alfred Oduor Odindo1 and Jacob Mashilo2, 1 Crop Science, School of Agricultural Earth and Environmental Sciences, University of KwaZulu-Natal, South Africa 2 Limpopo Department of Agriculture and Rural Development Towoomba Research Station, Private Bag X1615, Bela-Bela 0480, South Africa * Correspondence: [email protected]; Tel.: +27629810895 Abstract: Citron watermelon (Citrullus lanatus var. citroides) is an underexploited and un- der-researched crop species with potential to contribute to crop diversification in sub-Saharan Af- rica and beyond. The species is commonly cultivated in the drier parts of Southern Africa, mainly by smallholder farmers who maintain a wide range of landraces. Understanding the molecular and morpho-physiological basis for drought adaptation of Citron watermelon in these dry environ- ments can aid in screening local germplasm, identification of suitable traits for crop improvement and improving food system resilience among smallholder farmers by adding to crop diversifica- tion. This paper reviews literature on drought adaptation of C. lanatus spp. (C3 xerophytes), using the systematic review approach. The review discusses; (i) the potential role of citron watermelon in adding to crop diversification, (ii) alternative food uses and potential by-products that can be pro- cessed from citron watermelon and (iii) the role of Sub-Saharan farmers as key actors in conserving citron watermelon germplasm and biodiversity. Finally, the review provides a summary of signif- icant findings and identifies critical knowledge gaps for further research. -

Growing Citrus in the North Bay
Growing Citrus in the North Bay Steven Swain UC Cooperative Extension, Marin & Sonoma Counties (415) 473-4204 [email protected] http://cemarin.ucanr.edu The title is almost an oxymoron Where do citrus trees come from? . Southeast Asia . Burma (Myanmar) . Yunnan province of China . Northeast India In California, we’re used to being able to grow anything . But California’s famous for lots of climates in a small area Where is citrus Sacramento Valley: 0.4% commercially grown? Not here … San Joaquin Valley: 73% . There’s probably more than one reason for that Desert Valleys: 5% . Commercial citrus in Sacramento Valley is restricted to hot spots . Commercial grapefruit restricted to inland locations with water – Why? Coast: 12% . Citrus is a subtropical plant – It needs heat to produce sugar South Coast And Interior: 10% Citrus development periods Development Jan Feb Mar Apr May Jun Jul Aug Sept Oct Nov Dec prebloom shoot growth and leaf flush bloom petal fall, leaf drop (?) root growth fruit drop fruit development slow increase in size rapid increase in size maturation, slow increase … for navel oranges grown in San Joaquin County The average time of year for each development stage is shown in dark gray, less vigorous development is shown in light gray Note early drop (light gray), June drop (dark gray), and preharvest drop (light gray) Prebloom: All citrus except lemon essentially stop growing in California’s climate (variable due to weather) Note that maturation can extend into early May in some citrus varietals in some regions Table adapted from IPM for Citrus, 3rd ed., in turn from Lovatt, in prep From: Bower JP (2004) The pre- and postharvest application potential for CropSet and ISR2000 on citrus. -

Chemical Variability of Peel and Leaf Essential Oils in the Citrus Subgenus Papeda (Swingle) and Few Relatives
plants Article Chemical Variability of Peel and Leaf Essential Oils in the Citrus Subgenus Papeda (Swingle) and Few Relatives Clémentine Baccati 1, Marc Gibernau 1, Mathieu Paoli 1 , Patrick Ollitrault 2,3 ,Félix Tomi 1,* and François Luro 2 1 Laboratoire Sciences Pour l’Environnement, Equipe Chimie et Biomasse, Université de Corse—CNRS, UMR 6134 SPE, Route des Sanguinaires, 20000 Ajaccio, France; [email protected] (C.B.); [email protected] (M.G.); [email protected] (M.P.) 2 UMR AGAP Institut, Université Montpellier, CIRAD, INRAE, Institut Agro, 20230 San Giuliano, France; [email protected] (P.O.); [email protected] (F.L.) 3 CIRAD, UMR AGAP, 20230 San Giuliano, France * Correspondence: [email protected]; Tel.: +33-495-52-4122 Abstract: The Papeda Citrus subgenus includes several species belonging to two genetically distinct groups, containing mostly little-exploited wild forms of citrus. However, little is known about the potentially large and novel aromatic diversity contained in these wild citruses. In this study, we characterized and compared the essential oils obtained from peels and leaves from representatives of both Papeda groups, and three related hybrids. Using a combination of GC, GC-MS, and 13C-NMR spectrometry, we identified a total of 60 compounds in peel oils (PO), and 76 compounds in leaf oils (LO). Limonene was the major component in almost all citrus PO, except for C. micrantha and C. hystrix, where β-pinene dominated (around 35%). LO composition was more variable, with different Citation: Baccati, C.; Gibernau, M.; major compounds among almost all samples, except for two citrus pairs: C. -

New and Noteworthy Citrus Varieties Presentation
New and Noteworthy Citrus Varieties Citrus species & Citrus Relatives Hundreds of varieties available. CITRON Citrus medica • The citron is believed to be one of the original kinds of citrus. • Trees are small and shrubby with an open growth habit. The new growth and flowers are flushed with purple and the trees are sensitive to frost. • Ethrog or Etrog citron is a variety of citron commonly used in the Jewish Feast of Tabernacles. The flesh is pale yellow and acidic, but not very juicy. The fruits hold well on the tree. The aromatic fruit is considerably larger than a lemon. • The yellow rind is glossy, thick and bumpy. Citron rind is traditionally candied for use in holiday fruitcake. Ethrog or Etrog citron CITRON Citrus medica • Buddha’s Hand or Fingered citron is a unique citrus grown mainly as a curiosity. The six to twelve inch fruits are apically split into a varying number of segments that are reminiscent of a human hand. • The rind is yellow and highly fragrant at maturity. The interior of the fruit is solid rind with no flesh or seeds. • Fingered citron fruits usually mature in late fall to early winter and hold moderately well on the tree, but not as well as other citron varieties. Buddha’s Hand or Fingered citron NAVEL ORANGES Citrus sinensis • ‘Washington navel orange’ is also known • ‘Lane Late Navel’ was the first of a as the Bahia. It was imported into the number of late maturing Australian United States in 1870. navel orange bud sport selections of Washington navel imported into • These exceptionally delicious, seedless, California.