The Role of ICP Monitoring in Meningitis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fibromyalgia and Chronic Fatigue
Fibromyalgia and Chronic Fatigue Paul G. Swingle, Ph.D., R. Psych. My previous radio program and my present webcast program are both entitled “It’s all in your head!” How I came up with this title was listening to the discouraged patients that I routinely treated, and still treat, who have been told, by the doctors they have consulted, that the pain, discomfort, distress, debilitating fatigue, sleep disturbance and depression that they endure is “all in your head.” What this remark is meant to imply, of course, is that there is no physical cause of the condition and that the symptoms are manifestations of psychological factors. My response to these patients is that “of course it is in your head, where else would it be?” My remark however is not disparaging but rather indicates the actual location of many of the patients symptoms. The brain controls everything so all symptoms are associated with brain activity. The remarkable effectiveness of neurotherapy for the treatment of the symptoms associated with fibromyalgia and chronic fatigue is due to the fact that the brain functioning associated with the symptoms is treated. Although this derogatory sentiment about fibromyalgia patients was widespread among health care providers in the past, it is remarkable to me that it still persists with many doctors to this day. A few days ago I was surfing the internet health news feeds and was dismayed to find an item entitled “Fibromyalgia: A mental illness?” In this item was a quote from a Canadian neurologist stating that fibromyalgia was a term waiting for a definition. -

Lumbar Puncture (LP)
Lumbar puncture (LP) What is a lumbar puncture (LP)? Who will perform this test? An LP is a common and routine procedure, also A doctor trained in performing this procedure known as a spinal tap, where a very small will do the LP. A trained children’s nurse will hold needle is inserted through the base of the spine your infant in the appropriate position (which to collect a sample of the fluid surrounding the is lying on their side curled up in a tight ball). brain and spinal cord. This fluid is called Another team member may also be present to cerebrospinal fluid (CSF). help. Why do we need to do this? Can you be present during the LP? LP is the only way to confirm a case of Yes, you can tell the team that you would like to meningitis (swelling of the lining of the brain be present. However, many parents find it caused by an infection in the CSF). distressing to see any procedures being done on their baby and would rather not be present. This National guidelines strongly recommended that is perfectly understandable. Please let the team an LP is done alongside other routine know and they will usually agree to whatever investigations, such as blood and urine tests, works best for you but be aware that it is best to look for signs of infection if meningitis is to be quiet so the doctor can concentrate on the suspected. This is a routine and very important procedure. investigation. Is it a painful procedure? Getting a sample of CSF will help Drs to find out An LP is an uncomfortable procedure similar to if your child has meningitis and if what is a blood test or drip being inserted. -

Post-Lumbar Puncture Headache—Does Hydration Before Puncture Prevent Headache and Affect Cerebral Blood Flow?
Journal of Clinical Medicine Article Post-Lumbar Puncture Headache—Does Hydration before Puncture Prevent Headache and Affect Cerebral Blood Flow? Magdalena Nowaczewska 1,* , Beata Kukulska-Pawluczuk 2, Henryk Ka´zmierczak 3 and Katarzyna Pawlak-Osi ´nska 1 1 Department of Pathophysiology of Hearing and Balance, Ludwig Rydygier Collegium Medicum in Bydgoszcz Nicolaus Copernicus University, M. Curie 9, 85-090 Bydgoszcz, Poland; [email protected] 2 Department of Neurology, Ludwig Rydygier Collegium Medicum in Bydgoszcz Nicolaus Copernicus University, M. Curie 9, 85-090 Bydgoszcz, Poland; [email protected] 3 Department of Otolaryngology, Head and Neck Surgery, and Laryngological Oncology, Ludwik Rydygier Collegium Medicum in Bydgoszcz Nicolaus Copernicus University, M. Curie 9, 85-090 Bydgoszcz, Poland; [email protected] * Correspondence: [email protected]; Tel.: +48-52-585-4716 Received: 8 September 2019; Accepted: 15 October 2019; Published: 17 October 2019 Abstract: Headache is a common complication after diagnostic lumbar puncture (DLP). We aimed to check whether hydration before puncture influences the incidence of post-lumbar puncture headache (PLPH) and affects cerebral blood flow. Ninety-nine patients enrolled for puncture were assigned to a group with (n = 40) or without hydration (n = 59). In the hydration group, 1000 mL 0.9% NaCl was infused and a minimum of 1500 mL oral fluids was recommended within the 24 h before puncture. A Transcranial Doppler (TCD) was performed before and after DLP. Mean velocity (Vm) and pulsatility index (PI) were measured in the middle cerebral arteries (MCAs). PLPH occurred in 28 patients (28.2%): six (15.4%) from the hydrated and 22 (37.3%) from the non-hydrated group (p < 0.023). -

Vascular Dementia: an Overview of Current Challenges and Gaps
Stroke and Dementia: what research tells us UK Stroke Assembly 2016 JM Wardlaw University of Edinburgh NHS Lothian www.strokeassembly.org.uk #strokeassembly Definitions Stroke Dementia Sudden onset focal A syndrome where there neurological deficit which is deterioration in last more than 24 hours memory, thinking, attributable to a vascular behaviour such that the defect and has no other person is no longer able identifiable cause to perform everyday activities by themselves www.strokeassembly.org.uk #strokeassembly Stroke – the importance • Stroke, 16.9million new strokes per year, – A third are disabled and dependent – 2nd commonest cause of death – Commonest cause of dependency in adults Infarct, or ischaemic stroke Haemorrhagic stroke www.strokeassembly.org.uk #strokeassembly Cerebrovascular disease – the importance • ‘Silent’ cerebrovascular disease, even bigger issue – Up to 45% of 35.6million dementias – Cognitive impairment, mobility problems Normal White matter disease www.strokeassembly.org.uk #strokeassembly Dementia • 47.5 million people have dementia world wide • 7.7million new cases per year • Affects different people in different ways • Major cause of dependency and disability amongst older people • Alzheimer’s disease commonest cause – 60-70% of cases • Vascular dementia 2nd commonest – 25-45% of all cases www.strokeassembly.org.uk #strokeassembly Dementia: historical perspective 1800’s – Blood vessel diseases 1900’s – Alzheimers disease - dominant 1970’s – ‘Multi-infarct dementia’ – vascular 1990’s–2000’s - ‘Vascular -

TIA Vs CVA (STROKE)
Phone: 973.334.3443 Email: [email protected] NJPR.com TIA vs CVA (STROKE) What is the difference between a TIA and a stroke? Difference Between TIA and Stroke • Both TIA and stroke are due to poor blood supply to the brain. • Stroke is a medical emergency and it’s a life-threatening condition. • The symptoms of TIA and Stroke may be same but TIA symptoms will recover within 24 hours. TRANSIENT ISCHEMIC ATTACK ● Also known as: TIA, mini stroke 80 E. Ridgewood Avenue, 4th Floor Paramus, NJ 07652 TIA Causes ● A transient ischemic attack has the same origins as that of an ischemic stroke, the most common type of stroke. In an ischemic stroke, a clot blocks the blood supply to part of your brain. In a transient ischemic attack, unlike a stroke, the blockage is brief, and there is no permanent damage. ● The underlying cause of a TIA often is a buildup of cholesterol- containing fatty deposits called plaques (atherosclerosis) in an artery or one of its branches that supplies oxygen and nutrients to your brain. ● Plaques can decrease the blood flow through an artery or lead to the development of a clot. A blood clot moving to an artery that supplies your brain from another part of your body, most commonly from your heart, also may cause a TIA. CEREBROVASCULAR ACCIDENT/STROKE Page 2 When the brain’s blood supply is insufficient, a stroke occurs. Stroke symptoms (for example, slurring of speech or loss of function in an arm or leg) indicate a medical emergency. Without treatment, the brain cells quickly become impaired or die. -

DIAGNOSTICS and THERAPY of INCREASED INTRACRANIAL PRESSURE in ISCHEMIC STROKE
DIAGNOSTICS and THERAPY of INCREASED INTRACRANIAL PRESSURE in ISCHEMIC STROKE Erich Schmutzhard INNSBRUCK, AUSTRIA e - mail: erich.schmutzhard@i - med.ac.at Neuro - ICU Innsbruck Conflict of interest : Speaker‘s honoraria from ZOLL Medical and Honoraria for manuscripts from Pfizer Neuro - ICU Innsbruck OUTLINE Introduction Definition: ICP, CPP, PbtiO2, metabolic monitoring, lactate , pyruvate , brain temperature etc Epidemiology of ischemic stroke and ICP elevation in ischemic stroke ACM infarction and the role of collaterals and/ or lack of collaterals for ICP etc Hydrocephalus in posterioir fossa ( mainly cerebellar ) infarction Diagnosis of ICP etc Monitoring of ICP etc Therapeutic management : decompressive craniectomy – meta - analysis of DESTINY HAMLET und co deepening of analgosedation , ventilation , hyperventilation , osmotherapy , CPP - oriented th , MAP management , hypothermia , prophylactic normothermia , external ventricular drainage Neuro - ICU Innsbruck Introduction - Malignant cerebral edema following ischemic stroke is life threatening. - The pathophysiology of brain edema involves failure of the sodium - potassium adenosine triphosphatase pump and disruption of the blood - brain barrier, leading to cytotoxic edema and cellular death. - The Monro - Kellie doctrine clearlc states that - since the brain is encased in a finite space - increased intracranial pressure (ICP) due to cerebral edema can result in herniation through the foramen magnum and openings formed by the falx and tentorium. - Moreover, elevated ICP can cause secondary brain ischemia through decreased cerebral perfusion and blood flow, brain tissue hypoxia, and metabolic crisis. - Direct cerebrovascular compression caused by brain tissue shifting can lead to secondary infarction, especially in the territories of the anterior and posterior cerebral artery. - Tissue shifts can also stretch and tear cerebral vessels, causing intracranial hemorrhage such as Duret’s hemorrhage of the brainstem. -
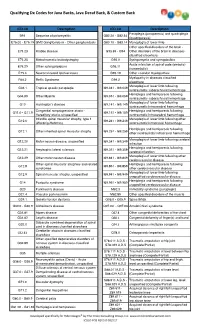
ICD-10 Backs
Qualifying Dx Codes for Java Backs, Java Decaf Back, & Custom Back ICD-10 Description ICD-10 Description Paraplegia (paraparesis) and quadriplegia B91 Sequelae of poliomyelitis G82.20 - G82.54 (quadriparesis) E75.00 - E75.19 GM2 Gangliosidosis - Other gangliosidosis G83.10 - G83.14 Monoplegia of lower limb Other specified disorders of the brain - E75.23 Krabbe disease G93.89 - G94 Other disorders of the brain in diseases classified elsewhere E75.25 Metachromatic leukodystrophy G95.0 Syringomyelia and syringobulbia Acute infarction of spinal code (embolic) E75.29 Other sphingolipidosis G95.11 (nonembolic) E75.4 Neuronal ceroid lipofuscinosis G95.19 Other vascular myelopathies Myelopathy in diseases classified F84.2 Rett's Syndrome G99.2 elsewhere Monoplegia of lower limb following G04.1 Tropical spastic paraplegia I69.041 - I69.049 nontraumatic subarachnoid hemorrhage Hemiplegia and hemiparesis following G04.89 Other Myelitis I69.051 - I69.059 nontraumatic subarachnoid hemorrhage Monoplegia of lower limb following G10 Huntington's disease I69.141 - I69.149 nontraumatic intracerebral hemorrhage Congenital nonprogressive ataxia - Hemiplegia and hemiparesis following G11.0 - G11.9 I69.151 - I69.159 Hereditary ataxia, unspecified nontraumatic intracerebral hemorrhage Infantile spinal muscular atrophy, type 1 Monoplegia of lower limb following other G12.0 I69.241 - I69.249 (Werdnig-Hoffman) nontraumatic intracranial hemorrhage Hemiplegia and hemiparesis following G12.1 Other inherited spinal muscular atrophy I69.251 - I69.259 other nontraumatic -

The Clinical Effect of Lumbar Puncture in Normal Pressure Hydrocephalus
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.45.1.64 on 1 January 1982. Downloaded from Journal of Neurology, Neurosurgery, and Psychiatry 1982;45:64-69 The clinical effect of lumbar puncture in normal pressure hydrocephalus C WIKKELS0, H ANDERSSON, C BLOMSTRAND, G LINDQVIST From the Department of Neurology and Neurosurgery, Sahlgren Hospital, University of Goteborg, Sweden SUMMARY Owing to all the difficulties involved in selecting patients with normal pressure hydro- cephalus for shunt-operation, a cerebrospinal fluid-tap-test (CSF-TT) is introduced. Psychometric and motor capacities of the patients are measured before and after lumbar puncture and removal of 40-50 ml CSF. Patients fulfilling criteria for normal pressure hydrocephalus were compared to patients with dementia and atrophy shown by computed tomography. Normal pressure hydro- cephaluspatients showed temporary improvement after lumbar puncture. The extent ofthe temporary improvement appeared to be well correlated with the improvement after shunt operation. Accord- ingly, the CSF-TT seems to be of value when selecting those patients who will probably benefit from a shunt operation. The syndrome of normal pressure hydrocephalus is pressure by lumbar puncture caused both subjective Protected by copyright. characterised by gait disturbance, progressive mental and objective temporary improvement in some deterioration and urinary incontinence.' Particularly patients with normal pressure hydrocephalus.10 16 17 noteworthy is the complex gait disturbance with Such patients also improved after CSF-shunting. spastic-ataxia, extrapyramidal components, and However, no details have been given about the commonly dyspraxia of gait.2 In addition, there is in amount of CSF which was drained or to what degree typical cases mental "slowing up" with lack of the CSF pressure was lowered. -

Ventriculoperitoneal Shunts in the Emergency Department: a Review
Open Access Review Article DOI: 10.7759/cureus.6857 Ventriculoperitoneal Shunts in the Emergency Department: A Review Michael Ferras 1 , Nicholas McCauley 1 , Trilok Stead 2 , Latha Ganti 3, 4, 5 , Bobby Desai 6 1. Emergency Medicine, Ocala Regional Medical Center, University of Central Florida, Ocala, USA 2. Emergency Medicine, Trinity Preparatory School, Winter Park, USA 3. Emergency Medicine, Envision Physician Services, Orlando, USA 4. Emergency Medicine, University of Central Florida College of Medicine/Hospital Corporation of America Graduate Medical Education Consortium of Greater Orlando, Orlando, USA 5. Emergency Medicine, Polk County Fire Rescue, Bartow, USA 6. Emergency Medicine, Ocala Regional Medical Center, University of Central Florida College of Medicine, Ocala, USA Corresponding author: Latha Ganti, [email protected] Abstract In this paper, we review the indications, complications, and pitfalls associated with ventriculoperitoneal (VP) shunts. As most VP shunt problems initially present to the emergency department, it is important for emergency physicians to be well-versed in managing them. In the article, the possible reasons for shunt failure are explored and summarized using an infographic. We also examine potential clinical presentations of VP shunt failure. Categories: Emergency Medicine, Neurosurgery Keywords: ventriculoperitoneal shunts Introduction And Background Emergency department physicians usually see a large number of patients with medical maladies managed by the aid of instrumentation or hardware such as a ventriculoperitoneal (VP) shunt. While patients have shunts placed for multiple reasons, it is important for emergency service providers to know how to evaluate, troubleshoot, and treat those with VP shunt complications. An estimated 30,000 VP shunt procedures are performed yearly in the United States [1]. -

Central Pain: Clinical and Physiological J Neurol Neurosurg Psychiatry: First Published As 10.1136/Jnnp.61.1.62 on 1 July 1996
626Journal ofNeurology, Neurosurgery, and Psychiatry 1996;61:62-69 Central pain: clinical and physiological J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.61.1.62 on 1 July 1996. Downloaded from characteristics David Bowsher Abstract spinothalamic pathway, its relays, or projec- Objectives-To study the clinical and tions may cause central pain,' and modern pathophysiological features of central radiological techniques have tended to con- pain due to damage to the CNS. firm this.9 '" Because of this, and of recent Methods-156 patients (mostly with considerations on pathophysiology,'5 '" the ischaemic strokes, some with infarct after condition is now known as "central poststroke subarachnoid haemorrhage and other pain (CPSP)"'.'7 Stroke is not the only condi- cerebral conditions; one with bulbar and tion causing such central pains of cerebral ori- others with spinal pathology) with central gin.' lxII 2' Furthermore, identical pains occur pain have been investigated clinically and after some spinal lesions.- 22 varying numbers instrumentally with respect to quantitative somatosensory perception thresholds and autonomic Patients function. Between 1983 and 1993, 156 patients have Results-Pain onset was immediate in a been referred to me, in whom a diagnosis of minority; and from a week or two up to central pain due to a cerebral or spinal lesion six years in > 60%. For those with supra- has been made. Of these, 112 (64 (57)%'Y spinal ischaemic lesions, the median age male) had a history of a stroke episode (cere- of onset was 59; dominant and non- brovascular accident, CVA patients), includ- dominant sides were equally affected. -

Stroke Intracranial Hypertension Cerebral Edema Roman Gardlík, MD, Phd
Stroke Intracranial hypertension Cerebral edema Roman Gardlík, MD, PhD. Institute of Pathological Physiology Institute of Molecular Biomedicine [email protected] Books • Silbernagl 356 • Other book 667 Brain • The most complex structure in the body • Anatomically • Functionally • Signals to and from various part of the body are controlled by very specific areas within the brain • Brain is more vulnerable to focal lesions than other organs • Renal infarct does not have a significant effect on kidney function • Brain infarct of the same size can produce complete paralysis on one side of the body Brain • 2% of body weight • Receives 1/6 of resting cardiac output • 20% of oxygen consumption Blood-brain barrier Mechanisms of brain injury • Various causes: • trauma • tumors • stroke • metabolic dysbalance • Common pathways of injury: • Hypoxia • Ischemia • Cerebral edema • Increased intracranial pressure Hypoxia • Deprivation of oxygen with maintained blood flow • Causes: • Exposure to reduced atmospheric pressure • Carbon monoxide poisoning • Severe anemia • Failure to ogygenate blood • Well tolerated, particularly if chronic • Neurons capable of anaerobic metabolism • Euphoria, listlessness, drowsiness, impaired problem solving • Acute and severe hypoxia – unconsciousness and convulsions • Brain anoxia can result to cardiac arrest Ischemia • Reduced blood flow • Focal / global ischemia • Energy sources (glucose and glycogen) are exhausted in 2 to 4 minutes • Cellular ATP stores are depleted in 4 to 5 minutes • 50% - 75% of energy is -

Idiopathic Intracranial Hypertension
IDIOPATHIC INTRACRANIAL HYPERTENSION William L Hills, MD Neuro-ophthalmology Oregon Neurology Associates Affiliated Assistant Professor Ophthalmology and Neurology Casey Eye Institute, OHSU No disclosures CASE - 19 YO WOMAN WITH HEADACHES X 3 MONTHS Headaches frontal PMHx: obesity Worse lying down Meds: takes ibuprofen for headaches Wake from sleep Pulsatile tinnitus x 1 month. Vision blacks out transiently when she bends over or sits down EXAMINATION Vision: 20/20 R eye, 20/25 L eye. Neuro: PERRL, no APD, EOMI, VF full to confrontation. Dilated fundoscopic exam: 360 degree blurring of disc margins in both eyes, absent SVP. Formal visual field testing: Enlargement of the blind spot, generalized constriction both eyes. MRI brain: Lumbar puncture: Posterior flattening of Opening pressure 39 the globes cm H20 Empty sella Normal CSF studies otherwise normal Headache improved after LP IDIOPATHIC INTRACRANIAL HYPERTENSION SYNDROME: Increased intracranial pressure without ventriculomegaly or mass lesion Normal CSF composition NOMENCLATURE Idiopathic intracranial hypertension (IIH) Benign intracranial hypertension Pseudotumor cerebri Intracranial hypertension secondary to… DIAGNOSTIC CRITERIA Original criteria have been updated to reflect new imaging modalities: 1492 Friedman and Jacobsen. Neurology 2002; 59: Symptoms and signs reflect only those of - increased ICP or papilledema 1495 Documented increased ICP during LP in lateral decubitus position Normal CSF composition No evidence of mass, hydrocephalus, structural