Genetic Variability and Its Relationship to Acanthamoeba
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Protistology Mitochondrial Genomes of Amoebozoa
Protistology 13 (4), 179–191 (2019) Protistology Mitochondrial genomes of Amoebozoa Natalya Bondarenko1, Alexey Smirnov1, Elena Nassonova1,2, Anna Glotova1,2 and Anna Maria Fiore-Donno3 1 Department of Invertebrate Zoology, Faculty of Biology, Saint Petersburg State University, 199034 Saint Petersburg, Russia 2 Laboratory of Cytology of Unicellular Organisms, Institute of Cytology RAS, 194064 Saint Petersburg, Russia 3 University of Cologne, Institute of Zoology, Terrestrial Ecology, 50674 Cologne, Germany | Submitted November 28, 2019 | Accepted December 10, 2019 | Summary In this mini-review, we summarize the current knowledge on mitochondrial genomes of Amoebozoa. Amoebozoa is a major, early-diverging lineage of eukaryotes, containing at least 2,400 species. At present, 32 mitochondrial genomes belonging to 18 amoebozoan species are publicly available. A dearth of information is particularly obvious for two major amoebozoan clades, Variosea and Tubulinea, with just one mitochondrial genome sequenced for each. The main focus of this review is to summarize features such as mitochondrial gene content, mitochondrial genome size variation, and presence or absence of RNA editing, showing if they are unique or shared among amoebozoan lineages. In addition, we underline the potential of mitochondrial genomes for multigene phylogenetic reconstruction in Amoebozoa, where the relationships among lineages are not fully resolved yet. With the increasing application of next-generation sequencing techniques and reliable protocols, we advocate mitochondrial -

The Intestinal Protozoa
The Intestinal Protozoa A. Introduction 1. The Phylum Protozoa is classified into four major subdivisions according to the methods of locomotion and reproduction. a. The amoebae (Superclass Sarcodina, Class Rhizopodea move by means of pseudopodia and reproduce exclusively by asexual binary division. b. The flagellates (Superclass Mastigophora, Class Zoomasitgophorea) typically move by long, whiplike flagella and reproduce by binary fission. c. The ciliates (Subphylum Ciliophora, Class Ciliata) are propelled by rows of cilia that beat with a synchronized wavelike motion. d. The sporozoans (Subphylum Sporozoa) lack specialized organelles of motility but have a unique type of life cycle, alternating between sexual and asexual reproductive cycles (alternation of generations). e. Number of species - there are about 45,000 protozoan species; around 8000 are parasitic, and around 25 species are important to humans. 2. Diagnosis - must learn to differentiate between the harmless and the medically important. This is most often based upon the morphology of respective organisms. 3. Transmission - mostly person-to-person, via fecal-oral route; fecally contaminated food or water important (organisms remain viable for around 30 days in cool moist environment with few bacteria; other means of transmission include sexual, insects, animals (zoonoses). B. Structures 1. trophozoite - the motile vegetative stage; multiplies via binary fission; colonizes host. 2. cyst - the inactive, non-motile, infective stage; survives the environment due to the presence of a cyst wall. 3. nuclear structure - important in the identification of organisms and species differentiation. 4. diagnostic features a. size - helpful in identifying organisms; must have calibrated objectives on the microscope in order to measure accurately. -

Acanthamoeba Spp., Balamuthia Mandrillaris, Naegleria Fowleri, And
MINIREVIEW Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris , Naegleria fowleri , and Sappinia diploidea Govinda S. Visvesvara1, Hercules Moura2 & Frederick L. Schuster3 1Division of Parasitic Diseases, National Center for Infectious Diseases, Atlanta, Georgia, USA; 2Division of Laboratory Sciences, National Center for Environmental Health, Centers for Disease Control and Prevention, Atlanta, Georgia, USA; and 3Viral and Rickettsial Diseases Laboratory, California Department of Health Services, Richmond, California, USA Correspondence: Govinda S. Visvesvara, Abstract Centers for Disease Control and Prevention, Chamblee Campus, F-36, 4770 Buford Among the many genera of free-living amoebae that exist in nature, members of Highway NE, Atlanta, Georgia 30341-3724, only four genera have an association with human disease: Acanthamoeba spp., USA. Tel.: 1770 488 4417; fax: 1770 488 Balamuthia mandrillaris, Naegleria fowleri and Sappinia diploidea. Acanthamoeba 4253; e-mail: [email protected] spp. and B. mandrillaris are opportunistic pathogens causing infections of the central nervous system, lungs, sinuses and skin, mostly in immunocompromised Received 8 November 2006; revised 5 February humans. Balamuthia is also associated with disease in immunocompetent chil- 2007; accepted 12 February 2007. dren, and Acanthamoeba spp. cause a sight-threatening infection, Acanthamoeba First published online 11 April 2007. keratitis, mostly in contact-lens wearers. Of more than 30 species of Naegleria, only one species, N. fowleri, causes an acute and fulminating meningoencephalitis in DOI:10.1111/j.1574-695X.2007.00232.x immunocompetent children and young adults. In addition to human infections, Editor: Willem van Leeuwen Acanthamoeba, Balamuthia and Naegleria can cause central nervous system infections in animals. Because only one human case of encephalitis caused by Keywords Sappinia diploidea is known, generalizations about the organism as an agent of primary amoebic meningoencephalitis; disease are premature. -

Legionella Shows a Diverse Secondary Metabolism Dependent on a Broad Spectrum Sfp-Type Phosphopantetheinyl Transferase
Legionella shows a diverse secondary metabolism dependent on a broad spectrum Sfp-type phosphopantetheinyl transferase Nicholas J. Tobias1, Tilman Ahrendt1, Ursula Schell2, Melissa Miltenberger1, Hubert Hilbi2,3 and Helge B. Bode1,4 1 Fachbereich Biowissenschaften, Merck Stiftungsprofessur fu¨r Molekulare Biotechnologie, Goethe Universita¨t, Frankfurt am Main, Germany 2 Max von Pettenkofer Institute, Ludwig-Maximilians-Universita¨tMu¨nchen, Munich, Germany 3 Institute of Medical Microbiology, University of Zu¨rich, Zu¨rich, Switzerland 4 Buchmann Institute for Molecular Life Sciences, Goethe Universita¨t, Frankfurt am Main, Germany ABSTRACT Several members of the genus Legionella cause Legionnaires’ disease, a potentially debilitating form of pneumonia. Studies frequently focus on the abundant number of virulence factors present in this genus. However, what is often overlooked is the role of secondary metabolites from Legionella. Following whole genome sequencing, we assembled and annotated the Legionella parisiensis DSM 19216 genome. Together with 14 other members of the Legionella, we performed comparative genomics and analysed the secondary metabolite potential of each strain. We found that Legionella contains a huge variety of biosynthetic gene clusters (BGCs) that are potentially making a significant number of novel natural products with undefined function. Surprisingly, only a single Sfp-like phosphopantetheinyl transferase is found in all Legionella strains analyzed that might be responsible for the activation of all carrier proteins in primary (fatty acid biosynthesis) and secondary metabolism (polyketide and non-ribosomal peptide synthesis). Using conserved active site motifs, we predict Submitted 29 June 2016 some novel compounds that are probably involved in cell-cell communication, Accepted 25 October 2016 Published 24 November 2016 differing to known communication systems. -

The Risk to Human Health from Free-Living Amoebae Interaction with Legionella in Drinking and Recycled Water Systems
THE RISK TO HUMAN HEALTH FROM FREE-LIVING AMOEBAE INTERACTION WITH LEGIONELLA IN DRINKING AND RECYCLED WATER SYSTEMS Dissertation submitted by JACQUELINE MARIE THOMAS BACHELOR OF SCIENCE (HONOURS) AND BACHELOR OF ARTS, UNSW In partial fulfillment of the requirements for the award of DOCTOR OF PHILOSOPHY in ENVIRONMENTAL ENGINEERING SCHOOL OF CIVIL AND ENVIRONMENTAL ENGINEERING FACULTY OF ENGINEERING MAY 2012 SUPERVISORS Professor Nicholas Ashbolt Office of Research and Development United States Environmental Protection Agency Cincinnati, Ohio USA and School of Civil and Environmental Engineering Faculty of Engineering The University of New South Wales Sydney, Australia Professor Richard Stuetz School of Civil and Environmental Engineering Faculty of Engineering The University of New South Wales Sydney, Australia Doctor Torsten Thomas School of Biotechnology and Biomolecular Sciences Faculty of Science The University of New South Wales Sydney, Australia ORIGINALITY STATEMENT '1 hereby declare that this submission is my own work and to the best of my knowledge it contains no materials previously published or written by another person, or substantial proportions of material which have been accepted for the award of any other degree or diploma at UNSW or any other educational institution, except where due acknowledgement is made in the thesis. Any contribution made to the research by others, with whom 1 have worked at UNSW or elsewhere, is explicitly acknowledged in the thesis. I also declare that the intellectual content of this thesis is the product of my own work, except to the extent that assistance from others in the project's design and conception or in style, presentation and linguistic expression is acknowledged.' Signed ~ ............................ -

Diagnosis of Infections Caused by Pathogenic Free-Living Amoebae
Virginia Commonwealth University VCU Scholars Compass Microbiology and Immunology Publications Dept. of Microbiology and Immunology 2009 Diagnosis of Infections Caused by Pathogenic Free- Living Amoebae Bruno da Rocha-Azevedo Virginia Commonwealth University Herbert B. Tanowitz Albert Einstein College of Medicine Francine Marciano-Cabral Virginia Commonwealth University Follow this and additional works at: http://scholarscompass.vcu.edu/micr_pubs Part of the Medicine and Health Sciences Commons Copyright © 2009 Bruno da Rocha-Azevedo et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Downloaded from http://scholarscompass.vcu.edu/micr_pubs/9 This Article is brought to you for free and open access by the Dept. of Microbiology and Immunology at VCU Scholars Compass. It has been accepted for inclusion in Microbiology and Immunology Publications by an authorized administrator of VCU Scholars Compass. For more information, please contact [email protected]. Hindawi Publishing Corporation Interdisciplinary Perspectives on Infectious Diseases Volume 2009, Article ID 251406, 14 pages doi:10.1155/2009/251406 Review Article Diagnosis of Infections Caused by Pathogenic Free-Living Amoebae Bruno da Rocha-Azevedo,1 Herbert B. Tanowitz,2 and Francine Marciano-Cabral1 1 Department of Microbiology and Immunology, Virginia Commonwealth University School of Medicine, Richmond, VA 23298, USA 2 Department of Pathology, Albert Einstein College of Medicine, Bronx, NY 10461, USA Correspondence should be addressed to Francine Marciano-Cabral, [email protected] Received 25 March 2009; Accepted 5 June 2009 Recommended by Louis M. Weiss Naegleria fowleri, Acanthamoeba spp., Balamuthia mandrillaris,andSappinia sp. -

Bacterial Brain Abscess in a Patient with Granulomatous Amebic Encephalitis
SVOA Neurology ISSN: 2753-9180 Case Report Bacterial Brain Abscess in a Patient with Granulomatous Amebic Encephalitis. A Misdiagnosis or Free-Living Amoeba Acting as Trojan Horse? Rolando Lovaton1* and Wesley Alaba1 1 Hospital Nacional Cayetano Heredia (Lima-Peru) *Corresponding Author: Dr. Rolando Lovaton, Neurosurgery Service-Hospital Nacional Cayetano Heredia, Avenida Honorio Delgado 262 San Martin de Porres, Lima-Peru Received: July 13, 2021 Published: July 24, 2021 Abstract Amebic encephalitis is a rare and devastating disease. Mortality rate is almost 90% of cases. Here is described a very rare case of bacterial brain abscess in a patient with recent diagnosis of granulomatous amebic encephalitis. Case De- scription: A 29-year-old woman presented with headache, right hemiparesis and tonic-clonic seizure. Patient was diag- nosed with granulomatous amebic encephalitis due to Acanthamoeba spp.; although, there was no improvement of symptoms in spite of stablished treatment. Three months after initial diagnosis, a brain MRI showed a ring-enhancing lesion in the left frontal lobe compatible with brain abscess. Patient was scheduled for surgical evacuation and brain abscess was confirmed intraoperatively. However, Gram staining of the purulent content showed gram-positive cocci. Patient improved headache and focal deficit after surgery. Conclusion: It is the first reported case of a patient with cen- tral nervous system infection secondary to Acanthamoeba spp. who presented a bacterial brain abscess in a short time. Keywords: amebic encephalitis; Acanthamoeba spp; bacterial brain abscess Introduction Free–living amoebae cause potentially fatal infection of central nervous system. Two clinical entities have been de- scribed for amebic encephalitis: primary amebic meningoencephalitis (PAM), and granulomatous amebic encephalitis (GAE). -

Acanthamoeba Castellanii
Int. J. Biol. Sci. 2018, Vol. 14 306 Ivyspring International Publisher International Journal of Biological Sciences 2018; 14(3): 306-320. doi: 10.7150/ijbs.23869 Research Paper Environmental adaptation of Acanthamoeba castellanii and Entamoeba histolytica at genome level as seen by comparative genomic analysis Victoria Shabardina1, Tabea Kischka1, Hanna Kmita2, Yutaka Suzuki3, Wojciech Maka owski1 1. Institute of Bioinformatics, University Münster, Niels-Stensen Strasse 14, Münster 48149, Germany ł 2. Laboratory of Bioenergetics, Institute of Molecular Biology and Biotechnology, Faculty of Biology, Adam Mickiewicz University 3. Department of Computational Biology and Medical Sciences, Graduate School of Frontier Sciences, The University of Tokyo, 5-1-5 Kashiwanoha, Kashiwa, Chiba 277-8562, Japan Corresponding author: [email protected] © Ivyspring International Publisher. This is an open access article distributed under the terms of the Creative Commons Attribution (CC BY-NC) license (https://creativecommons.org/licenses/by-nc/4.0/). See http://ivyspring.com/terms for full terms and conditions. Received: 2017.11.15; Accepted: 2017.12.30; Published: 2018.02.12 Abstract Amoebozoans are in many aspects interesting research objects, as they combine features of single-cell organisms with complex signaling and defense systems, comparable to multicellular organisms. Acanthamoeba castellanii is a cosmopolitan species and developed diverged feeding abilities and strong anti-bacterial resistance; Entamoeba histolytica is a parasitic amoeba, who underwent massive gene loss and its genome is almost twice smaller than that of A. castellanii. Nevertheless, both species prosper, demonstrating fitness to their specific environments. Here we compare transcriptomes of A. castellanii and E. histolytica with application of orthologs’ search and gene ontology to learn how different life strategies influence genome evolution and restructuring of physiology. -

Developing Novel Therapeutic Agents for Acanthamoeba Infection and Investigating the Process of Encystment
Developing novel therapeutic agents for Acanthamoeba infection and investigating the process of encystment Anas Abdullah Hamad (BSc, MSc) A thesis submitted in partial fulfilment of the requirements of the University of Wolverhampton for the degree of Doctor of Philosophy June 2020 Declaration This work or any part thereof has not previously been presented in any form to the University or to any other body whether for the purposes of assessment, publication or for any other purpose (unless otherwise indicated in page 3). Save for any express acknowledgements, references and/or bibliographies cited in the work, I confirm that the intellectual content of the work is the result of my own efforts and of no other person. The right of Anas Abdullah Hamad to be identified as author of this work is asserted in accordance with ss.77 and 78 of the Copyright, Designs and Patents Act 1988. At this date copyright is owned by the author. Signature………………………………………. Date……………………………………………. 15/10/2020 2 List of posters and publication related to the work presented in this thesis: Heaselgrave, W., Hamad, A., Coles, S. and Hau, S., 2019. In Vitro Evaluation of the Inhibitory Effect of Topical Ophthalmic Agents on Acanthamoeba Viability. Translational vision science & technology, 8(5), pp.17-17. Manuscript published. Hamad, A. and Heaselgrave, W., 2017. Developing novel treatments for the blinding protozoan eye infection Acanthamoeba keratitis. Proceedings of the Internal Annual Research Symposium, Poster no. 23, University of Wolverhampton, UK. Hamad, A. and Heaselgrave, W., 2018. Developing new treatments and optimising existing treatment strategies for the corneal infection Acanthamoeba keratitis. -

This Thesis Has Been Submitted in Fulfilment of the Requirements for a Postgraduate Degree (E.G
This thesis has been submitted in fulfilment of the requirements for a postgraduate degree (e.g. PhD, MPhil, DClinPsychol) at the University of Edinburgh. Please note the following terms and conditions of use: This work is protected by copyright and other intellectual property rights, which are retained by the thesis author, unless otherwise stated. A copy can be downloaded for personal non-commercial research or study, without prior permission or charge. This thesis cannot be reproduced or quoted extensively from without first obtaining permission in writing from the author. The content must not be changed in any way or sold commercially in any format or medium without the formal permission of the author. When referring to this work, full bibliographic details including the author, title, awarding institution and date of the thesis must be given. Protein secretion and encystation in Acanthamoeba Alvaro de Obeso Fernández del Valle Doctor of Philosophy The University of Edinburgh 2018 Abstract Free-living amoebae (FLA) are protists of ubiquitous distribution characterised by their changing morphology and their crawling movements. They have no common phylogenetic origin but can be found in most protist evolutionary branches. Acanthamoeba is a common FLA that can be found worldwide and is capable of infecting humans. The main disease is a life altering infection of the cornea named Acanthamoeba keratitis. Additionally, Acanthamoeba has a close relationship to bacteria. Acanthamoeba feeds on bacteria. At the same time, some bacteria have adapted to survive inside Acanthamoeba and use it as transport or protection to increase survival. When conditions are adverse, Acanthamoeba is capable of differentiating into a protective cyst. -

EVALUATION of the P45 MOBILE INTEGRATIVE ELEMENT and ITS ROLE IN
EVALUATION OF THE p45 MOBILE INTEGRATIVE ELEMENT AND ITS ROLE IN Legionella pneumophila VIRULENCE A Dissertation by LANETTE M. CHRISTENSEN Submitted to the Office of Graduate and Professional Studies of Texas A&M University in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY Chair of Committee, Jeffrey D. Cirillo Committee Members, James Samuel Jon Skare Farida Sohrabji Head of Program, Warren Zimmer May 2018 Major Subject: Medical Sciences Copyright 2018 Lanette Christensen ABSTRACT Legionella pneumophila are aqueous environmental bacilli that live within protozoal species and cause a potentially fatal form of pneumonia called Legionnaires’ disease. Not all L. pneumophila strains have the same capacity to cause disease in humans. The majority of strains that cause clinically relevant Legionnaires’ disease harbor the p45 mobile integrative genomic element. Contribution of the p45 element to L. pneumophila virulence and ability to withstand environmental stress were addressed in this study. The L. pneumophila Philadelphia-1 (Phil-1) mobile integrative element, p45, was transferred into the attenuated strain Lp01 via conjugation, designating p45 an integrative conjugative element (ICE). The resulting trans-conjugate, Lp01+p45, was compared with strains Phil-1 and Lp01 to assess p45 in virulence using a guinea pig model infected via aerosol. The p45 element partially recovered the loss of virulence in Lp01 compared to that of Phil-1 evident in morbidity, mortality, and bacterial burden in the lungs at the time of death. This phenotype was accompanied by enhanced expression of type II interferon in the lungs and spleens 48 hours after infection, independent of bacterial burden. -
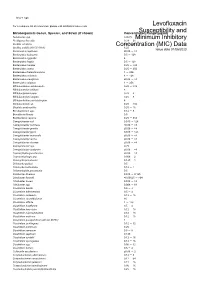
Susceptibility and Resistance Data
toku-e logo For a complete list of references, please visit antibiotics.toku-e.com Levofloxacin Microorganism Genus, Species, and Strain (if shown) Concentration Range (μg/ml)Susceptibility and Aeromonas spp. 0.0625 Minimum Inhibitory Alcaligenes faecalis 0.39 - 25 Bacillus circulans Concentration0.25 - 8 (MIC) Data Bacillus subtilis (ATCC 6051) 6.25 Issue date 01/06/2020 Bacteroides capillosus ≤0.06 - >8 Bacteroides distasonis 0.5 - 128 Bacteroides eggerthii 4 Bacteroides fragilis 0.5 - 128 Bacteroides merdae 0.25 - >32 Bacteroides ovatus 0.25 - 256 Bacteroides thetaiotaomicron 1 - 256 Bacteroides uniformis 4 - 128 Bacteroides ureolyticus ≤0.06 - >8 Bacteroides vulgatus 1 - 256 Bifidobacterium adolescentis 0.25 - >32 Bifidobacterium bifidum 8 Bifidobacterium breve 0.25 - 8 Bifidobacterium longum 0.25 - 8 Bifidobacterium pseudolongum 8 Bifidobacterium sp. 0.25 - >32 Bilophila wadsworthia 0.25 - 16 Brevibacterium spp. 0.12 - 8 Brucella melitensis 0.5 Burkholderia cepacia 0.25 - 512 Campylobacter coli 0.015 - 128 Campylobacter concisus ≤0.06 - >8 Campylobacter gracilis ≤0.06 - >8 Campylobacter jejuni 0.015 - 128 Campylobacter mucosalis ≤0.06 - >8 Campylobacter rectus ≤0.06 - >8 Campylobacter showae ≤0.06 - >8 Campylobacter spp. 0.25 Campylobacter sputorum ≤0.06 - >8 Capnocytophaga ochracea ≤0.06 - >8 Capnocytophaga spp. 0.006 - 2 Chlamydia pneumonia 0.125 - 1 Chlamydia psittaci 0.5 Chlamydia trachomatis 0.12 - 1 Chlamydophila pneumonia 0.5 Citrobacter diversus 0.015 - 0.125 Citrobacter freundii ≤0.00625 - >64 Citrobacter koseri 0.015 -