NSJ-Spine January 2004
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Chondrocyte Channelome: a Novel Ion Channel Candidate in the Pathogenesis of Pectus Deformities
Old Dominion University ODU Digital Commons Biological Sciences Theses & Dissertations Biological Sciences Summer 2017 The Chondrocyte Channelome: A Novel Ion Channel Candidate in the Pathogenesis of Pectus Deformities Anthony J. Asmar Old Dominion University, [email protected] Follow this and additional works at: https://digitalcommons.odu.edu/biology_etds Part of the Biology Commons, Molecular Biology Commons, and the Physiology Commons Recommended Citation Asmar, Anthony J.. "The Chondrocyte Channelome: A Novel Ion Channel Candidate in the Pathogenesis of Pectus Deformities" (2017). Doctor of Philosophy (PhD), Dissertation, Biological Sciences, Old Dominion University, DOI: 10.25777/pyha-7838 https://digitalcommons.odu.edu/biology_etds/19 This Dissertation is brought to you for free and open access by the Biological Sciences at ODU Digital Commons. It has been accepted for inclusion in Biological Sciences Theses & Dissertations by an authorized administrator of ODU Digital Commons. For more information, please contact [email protected]. THE CHONDROCYTE CHANNELOME: A NOVEL ION CHANNEL CANDIDATE IN THE PATHOGENESIS OF PECTUS DEFORMITIES by Anthony J. Asmar B.S. Biology May 2010, Virginia Polytechnic Institute M.S. Biology May 2013, Old Dominion University A Dissertation Submitted to the Faculty of Old Dominion University in Partial Fulfillment of the Requirements for the Degree of DOCTOR OF PHILOSOPHY BIOMEDICAL SCIENCES OLD DOMINION UNIVERSITY August 2017 Approved by: Christopher Osgood (Co-Director) Michael Stacey (Co-Director) Lesley Greene (Member) Andrei Pakhomov (Member) Jing He (Member) ABSTRACT THE CHONDROCYTE CHANNELOME: A NOVEL ION CHANNEL CANDIDATE IN THE PATHOGENESIS OF PECTUS DEFORMITIES Anthony J. Asmar Old Dominion University, 2017 Co-Directors: Dr. Christopher Osgood Dr. Michael Stacey Costal cartilage is a type of rod-like hyaline cartilage connecting the ribs to the sternum. -

Transcriptomic Profiling of Ca Transport Systems During
cells Article Transcriptomic Profiling of Ca2+ Transport Systems during the Formation of the Cerebral Cortex in Mice Alexandre Bouron Genetics and Chemogenomics Lab, Université Grenoble Alpes, CNRS, CEA, INSERM, Bâtiment C3, 17 rue des Martyrs, 38054 Grenoble, France; [email protected] Received: 29 June 2020; Accepted: 24 July 2020; Published: 29 July 2020 Abstract: Cytosolic calcium (Ca2+) transients control key neural processes, including neurogenesis, migration, the polarization and growth of neurons, and the establishment and maintenance of synaptic connections. They are thus involved in the development and formation of the neural system. In this study, a publicly available whole transcriptome sequencing (RNA-Seq) dataset was used to examine the expression of genes coding for putative plasma membrane and organellar Ca2+-transporting proteins (channels, pumps, exchangers, and transporters) during the formation of the cerebral cortex in mice. Four ages were considered: embryonic days 11 (E11), 13 (E13), and 17 (E17), and post-natal day 1 (PN1). This transcriptomic profiling was also combined with live-cell Ca2+ imaging recordings to assess the presence of functional Ca2+ transport systems in E13 neurons. The most important Ca2+ routes of the cortical wall at the onset of corticogenesis (E11–E13) were TACAN, GluK5, nAChR β2, Cav3.1, Orai3, transient receptor potential cation channel subfamily M member 7 (TRPM7) non-mitochondrial Na+/Ca2+ exchanger 2 (NCX2), and the connexins CX43/CX45/CX37. Hence, transient receptor potential cation channel mucolipin subfamily member 1 (TRPML1), transmembrane protein 165 (TMEM165), and Ca2+ “leak” channels are prominent intracellular Ca2+ pathways. The Ca2+ pumps sarco/endoplasmic reticulum Ca2+ ATPase 2 (SERCA2) and plasma membrane Ca2+ ATPase 1 (PMCA1) control the resting basal Ca2+ levels. -

Genetic Mapping of ASIC4 and Contrasting Phenotype to Asic1a in Modulating Innate Fear and Anxiety
European Journal of Neuroscience, Vol. 41, pp. 1553–1568, 2015 doi:10.1111/ejn.12905 BEHAVIORAL NEUROSCIENCE Genetic mapping of ASIC4 and contrasting phenotype to ASIC1a in modulating innate fear and anxiety Shing-Hong Lin,1,2 Ya-Chih Chien,2 Wei-Wei Chiang,3 Yan-Zhen Liu,3 Cheng-Chang Lien4 and Chih-Cheng Chen1,2,3 1Graduate institute of Life Sciences, National Defense Medical Center, Taipei, Taiwan 2Institute of Biomedical Sciences, Academia Sinica, Taipei 115, Taiwan 3Taiwan Mouse Clinic-National Comprehensive Mouse Phenotyping and Drug Testing Center, Academia Sinica, Taipei, Taiwan 4Institute of Neuroscience, National Yang-Ming University, Taipei, Taiwan Keywords: ASIC, behavioral phenotyping, Cre, interneuron, knockout mice Abstract Although ASIC4 is a member of the acid-sensing ion channel (ASIC) family, we have limited knowledge of its expression and physiological function in vivo. To trace the expression of this ion channel, we generated the ASIC4-knockout/CreERT2-knockin (Asic4CreERT2) mouse line. After tamoxifen induction in the Asic4CreERT2::CAG-STOPfloxed-Td-tomato double transgenic mice, we mapped the expression of ASIC4 at the cellular level in the central nervous system (CNS). ASIC4 was expressed in many brain regions, including the olfactory bulb, cerebral cortex, striatum, hippocampus, amygdala, thalamus, hypothalamus, brain stem, cer- ebellum, spinal cord and pituitary gland. Colocalisation studies further revealed that ASIC4 was expressed mainly in three types of cells in the CNS: (i) calretinin (CR)-positive and/or vasoactive intestine peptide (VIP)-positive interneurons; (ii) neural/glial anti- gen 2 (NG2)-positive glia, also known as oligodendrocyte precursor cells; and (iii) cerebellar granule cells. To probe the possible role of ASIC4, we hypothesised that ASIC4 could modulate the membrane expression of ASIC1a and thus ASIC1a signaling in vivo. -

Ion Channels
UC Davis UC Davis Previously Published Works Title THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: Ion channels. Permalink https://escholarship.org/uc/item/1442g5hg Journal British journal of pharmacology, 176 Suppl 1(S1) ISSN 0007-1188 Authors Alexander, Stephen PH Mathie, Alistair Peters, John A et al. Publication Date 2019-12-01 DOI 10.1111/bph.14749 License https://creativecommons.org/licenses/by/4.0/ 4.0 Peer reviewed eScholarship.org Powered by the California Digital Library University of California S.P.H. Alexander et al. The Concise Guide to PHARMACOLOGY 2019/20: Ion channels. British Journal of Pharmacology (2019) 176, S142–S228 THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: Ion channels Stephen PH Alexander1 , Alistair Mathie2 ,JohnAPeters3 , Emma L Veale2 , Jörg Striessnig4 , Eamonn Kelly5, Jane F Armstrong6 , Elena Faccenda6 ,SimonDHarding6 ,AdamJPawson6 , Joanna L Sharman6 , Christopher Southan6 , Jamie A Davies6 and CGTP Collaborators 1School of Life Sciences, University of Nottingham Medical School, Nottingham, NG7 2UH, UK 2Medway School of Pharmacy, The Universities of Greenwich and Kent at Medway, Anson Building, Central Avenue, Chatham Maritime, Chatham, Kent, ME4 4TB, UK 3Neuroscience Division, Medical Education Institute, Ninewells Hospital and Medical School, University of Dundee, Dundee, DD1 9SY, UK 4Pharmacology and Toxicology, Institute of Pharmacy, University of Innsbruck, A-6020 Innsbruck, Austria 5School of Physiology, Pharmacology and Neuroscience, University of Bristol, Bristol, BS8 1TD, UK 6Centre for Discovery Brain Science, University of Edinburgh, Edinburgh, EH8 9XD, UK Abstract The Concise Guide to PHARMACOLOGY 2019/20 is the fourth in this series of biennial publications. The Concise Guide provides concise overviews of the key properties of nearly 1800 human drug targets with an emphasis on selective pharmacology (where available), plus links to the open access knowledgebase source of drug targets and their ligands (www.guidetopharmacology.org), which provides more detailed views of target and ligand properties. -
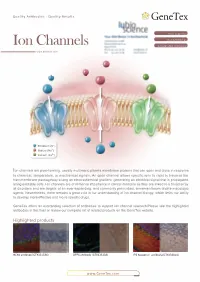
Ion Channels Accelerated D1scovery
Quality Antibodies · Quality Results $-GeneTex Your Expertise Our Antibod1es Ion Channels Accelerated D1scovery --------- www.genetex.com Potassium (K+ ) Sodium (Na+ ) Calcium (Ca2+ ) Ion channels are pore-forming, usually multimeric plasma membrane proteins that can open and close in response to chemical, temperature, or mechanical signals. An open channel allows specific ions to rapid ly traverse the transmembrane passageway a long an electrochemical gradient, generating an electrical signal that is propagated along excitable cells. Ion channels are of immense importance in clinical medicine as they are linked to a broad array of disorders and are targets of an ever-expanding, and commonly prescribed, armamentarium of pharmacologic agents. Nevertheless, there remains a great void in our understanding of ion channel biology, which limits our ability to develop more effective andmo re specific drugs. GeneTex offers an outstanding selection of antibodies to support ion channel research.Please see the highlighted antibodies in this flyer or review our complete list of related products on the GeneTex website. Highlighted products HCNl antibody (GTX131334} DPP6 antibody (GTX133338} IP3 Receptor I antibody (GTX133104} (...___ ___w _w _w_ ._G_e_n_e_T_e_x_._c_o_m_ _____,) ` a n a 匱 。 'ot I: . ·""·,, " . 鼴 丶•' · ,' , `> ,,, 丶 B -- ,:: ·-- o .i :' T. 、 冨 ,. \\.◄ .•}' r<• .. , -·•Jt.<_,,,, . ◄ · ◄ / .Jj -~~ 盆;i . '. ., CACNB4 (GTX100202) VGluTl antibody (GTX133148) P2X7一 antibody (GTX104288) Cavl.2 antibody (GTX54754) Cav2.1 antibody (GTX54753) -
A Variant of ASIC2 Mediates Sodium Retention in Nephrotic Syndrome
A variant of ASIC2 mediates sodium retention in nephrotic syndrome Marc Fila, … , Gilles Crambert, Alain Doucet JCI Insight. 2021. https://doi.org/10.1172/jci.insight.148588. Research In-Press Preview Nephrology Graphical abstract Find the latest version: https://jci.me/148588/pdf 1 A variant of ASIC2 mediates sodium retention in nephrotic syndrome 1,2Marc Fila *, 1,2Ali Sassi*, 1,2Gaëlle Brideau, 1,2Lydie Cheval, 1,2 Luciana Morla, 1,2Pascal Houillier, 1,2Christine Walter, 1,2Michel Gennaoui, 1,2Laure Collignon L, 1,2Mathilde Keck, 1,2Gabrielle Planelles, 1,2Naziha Bakouh, 3Michel Peuchmaur, 4Georges Deschênes, 5Ignacio Anegon, 5Séverine Remy, 6Bruno Vogt, 1,2 Gilles Crambert#, 1,2Alain Doucet 1Centre de Recherche des Cordeliers, Sorbonne Universités, INSERM, Université de Paris, Laboratoire de Physiologie Rénale et Tubulopathies, F-75006, Paris, France 2CNRS, ERL8228, F-75006, Paris, France 3Cytology and Pathology DePartment, Robert Debré HosPital, F-75019, Paris, France 4Pediatric NePhrology DePartment, Robert Debré HosPital, F-75019, Paris, France 5INSERM UMR 1064, Centre de Recherches en TransPlantation et Immunologie (CRTI), Transgenic Rats ImmunoPhenomic facility, Nantes, France. 6DePartment of NePhrology and HyPertension, InselsPital, Bern University HosPital, CH- 3010, Bern, Switzerland Present address: Marc Fila, Pediatric NePhrology DePartment, HôPital Arnaud de Villeneuve Institut de Génomique Fonctionnelle UMR9023 CNRS U661 INSERM, MontPellier, France; Ali Sassi, DePartment of Cellular Physiology and Metabolism, University -

Acid-Sensing Ion Channels Emerged Over 600 Mya and Are Conserved Throughout the Deuterostomes
Acid-sensing ion channels emerged over 600 Mya and are conserved throughout the deuterostomes Timothy Lynagha,1, Yana Mikhalevab, Janne M. Coldinga, Joel C. Gloverb,c, and Stephan A. Plessa aDepartment of Drug Design and Pharmacology, Center for Biopharmaceuticals, University of Copenhagen, 2100 Copenhagen, Denmark; bSars International Centre for Marine Molecular Biology, University of Bergen, 5006 Bergen, Norway; and cDepartment of Molecular Medicine, University of Oslo, 0372 Oslo, Norway Edited by Richard W. Aldrich, The University of Texas at Austin, Austin, TX, and approved June 28, 2018 (received for review April 17, 2018) Acid-sensing ion channels (ASICs) are proton-gated ion channels physiology in more diverse animal lineages, and that we should broadly expressed in the vertebrate nervous system, converting therefore find ASICs in nonvertebrate genomes. However, pre- decreased extracellular pH into excitatory sodium current. ASICs vious studies suggest that ASICs are not readily identifiable were previously thought to be a vertebrate-specific branch of the through sequence analyses alone (9). This is likely due to our DEG/ENaC family, a broadly conserved but functionally diverse poor understanding of the molecular determinants of proton family of channels. Here, we provide phylogenetic and experi- sensitivity, notwithstanding the finding that the mutation of several protonatable side chains in the extracellular domain de- mental evidence that ASICs are conserved throughout deutero- – stome animals, showing that ASICs evolved over 600 million years creases proton sensitivity of ASICs (18 22). ago. We also provide evidence of ASIC expression in the central Here, we sought to illuminate the evolutionary emergence of ASICs and the molecular basis of proton sensing. -

Opioid-Mediated Modulation of Acid-Sensing Ion Channel Currents in Adult Rat Sensory Neurons
1521-0111/95/5/519–527$35.00 https://doi.org/10.1124/mol.118.114918 MOLECULAR PHARMACOLOGY Mol Pharmacol 95:519–527, May 2019 Copyright ª 2019 by The American Society for Pharmacology and Experimental Therapeutics Opioid-Mediated Modulation of Acid-Sensing Ion Channel Currents in Adult Rat Sensory Neurons Malgorzata Zaremba and Victor Ruiz-Velasco Ruiz-Velasco Laboratory, Department of Anesthesiology and Perioperative Medicine, Penn State College of Medicine, Hershey, Pennsylvania Received October 16, 2018; accepted February 20, 2019 Downloaded from ABSTRACT Muscle ischemia, associated with peripheral artery disease resulted in potentiation of the sustained ASIC currents. On the (PAD), leads to the release of proinflammatory mediators that other hand, the potentiation was not observed in DRG neurons decrease extracellular pH and trigger the activation of proton- from ASIC3 knockout rats. Further, the enhancement of the activated acid-sensing ion channels (ASIC). Claudication pain, ASIC currents was resistant to pertussis toxin treatment, suggesting linked with low blood flow, can be partially relieved by endogenous that Gai/Gao G-proteins are not involved. Additionally, the opioid peptide release. However, we previously reported that potentiation of sustained ASIC currents was greater in DRG molpharm.aspetjournals.org sustained ASIC currents in dorsal root ganglion (DRG) neurons neurons isolated from rats with ligated femoral arteries (a were enhanced by naturally occurring endomorphin-1 and -2 modelofPAD).Theeffectofallthree opioids on the transient opioid peptides, indicating a role of opioid involvement in ASIC peak current was mixed (increase, decrease, no effect). The hyperalgesia. The present study examined whether clinically inhibitory action appears to be mediated by the presence of ASIC1 employed synthetic (fentanyl, remifentanil) and the semisyn- isoform, while the potentiating effect is primarily due to ASIC3 thetic opioid (oxycodone) would also potentiate sustained isoform expression. -

(TRP) and Acid-Sensing Ion Channels (Asics) in the Sensory Organs of Adult Zebrafish
Chapter 6 Transient-Receptor Potential (TRP) and Acid-Sensing Ion Channels (ASICs) in the Sensory Organs of Adult Zebrafish AntoninoAntonino Germanà, Germanà, Juan D.D. Muriel, Muriel, Ramón Cobo,Ramón Cobo, OliviaOlivia García-Suárez, García-Suárez, Juan CoboJuan Cobo and José A. VegaA. Vega Additional information is available at the end of the chapter http://dx.doi.org/10.5772/intechopen.74492 Abstract Sensory information from the aquatic environment is required for life and survival of zebrafish. Changes in the environment are detected by specialized sensory cells that convert different types of stimuli into electric energy, thus originating an organ-specific transduction. Ion channels are at the basis of each sensory modality and are responsible or are required for detecting thermal, chemical, or mechanical stimuli but also for more complex sensory processes as hearing, olfaction, taste, or vision. The capacity of the sen- sory cells to preferentially detect a specific stimulus is the result of a characteristic com- bination of different ion channels. This chapter summarizes the current knowledge about the occurrence and localization of ion channels in sensory organs of zebrafish belonging to the superfamilies of transient-receptor potential and acid-sensing ion channels that are involved in different qualities of sensibility superfamilies in the sensory organs of zebraf- ish. This animal model is currently used to study some human pathologies in which ion channels are involved. Furthermore, zebrafish is regarded as an ideal model to study in vivo the transient-receptor potential ion channels. Keywords: sensory organs, sensibility, transient-receptor potential ion channels, acid-sensing ion channels, zebrafish 1. -

Acetylcholine Acetylcholine Receptors Acid-Sensing Ion Channels
Acid-Sensing Ion Channels 1 degenerin 1 (MDEG1), brain sodium channel 1 Acetylcholine (BNC1, BNaC1); ASIC2b: mammalian degenerin 2 (MDEG2); ASIC3: dorsal-root acid-sensing ion channel (DRASIC) Synonyms ACh Definition Acid-Sensing Ion Channels (ASICs) are membrane Definition protein complexes that form depolarizing ion channels Acetylcholine is the chemical mediator in the synapse present on peripheral and/or central neurons. These of a motor endplate. The electrical signal of the channels are opened by extracellular protons. Their acti- motor nerve terminal causes release of many packets vation induces action potential triggering on neurons of acetylcholine. The packets are released into the after an extracellular pH decrease to acidic values. Such synaptic cleft where receptors in the postjunctional tissue acidosis occurs during inflammation or ischemia, membrane of the striated muscle fiber membrane and is a major source of pain. convert the chemical signal to an electrical signal (a propagated action potential) that can produce muscle Characteristics contractile activity. Normally, an occasional acetylcho- ASICs are membrane protein complexes formed by line packet is released spontaneously by the nerve four subunits among the six characterized isoforms terminal without a nerve signal. Each packet produces a (Fig. 1). The isoforms are coded by four different miniature endplate potential in the muscle fiber, but its genes, two of them spliced in two variants: ASIC1a and amplitude is too small to be propagated. Myofascial ASIC1b, ASIC2a and ASIC2b, ASIC3 and ASIC4 trigger points are associated with excessive sponta- (Chen et al. 1998; Garcia-Anoveros et al. 1997; neous release of acetylcholine packets in affected Grunder et al. -

Pain-Causing Venom Peptides: Insights Into Sensory Neuron Pharmacology
toxins Review Pain-Causing Venom Peptides: Insights into Sensory Neuron Pharmacology Sina Jami 1, Andelain Erickson 2,3, Stuart M. Brierley 2,3 and Irina Vetter 1,4,* ID 1 Institute for Molecular Bioscience, the University of Queensland, St. Lucia, QLD 4072, Australia; [email protected] 2 Centre for Nutrition and Gastrointestinal Diseases, Discipline of Medicine, University of Adelaide, South Australian Health and Medical Research Institute (SAHMRI), North Terrace, Adelaide, South Australia 5000, Australia; [email protected] (A.E.); stuart.brierley@flinders.edu.au (S.M.B.) 3 Visceral Pain Research Group, Human Physiology, Centre for Neuroscience, College of Medicine and Public Health, Flinders University, Bedford Park, South Australia 5042, Australia 4 School of Pharmacy, The University of Queensland, Woolloongabba, QLD 4103, Australia * Correspondence: [email protected]; Tel.: +61-7-3346-2660 Received: 10 November 2017; Accepted: 20 December 2017; Published: 27 December 2017 Abstract: Venoms are produced by a wide variety of species including spiders, scorpions, reptiles, cnidarians, and fish for the purpose of harming or incapacitating predators or prey. While some venoms are of relatively simple composition, many contain hundreds to thousands of individual components with distinct pharmacological activity. Pain-inducing or “algesic” venom compounds have proven invaluable to our understanding of how physiological nociceptive neural networks operate. In this review, we present an overview of some of the diverse nociceptive pathways that can be modulated by specific venom components to evoke pain. Keywords: animal venom; pain; ASIC; sodium channel; TRP channel; pore forming toxin 1. Venoms and Their Pharmacological Effects Venoms can be defined broadly as toxins secreted by an animal for the purpose of harming or incapacitating predators or prey. -

SF3B1-Mutated Chronic Lymphocytic Leukemia Shows Evidence Of
SF3B1-mutated chronic lymphocytic leukemia shows evidence of NOTCH1 pathway activation including CD20 downregulation by Federico Pozzo, Tamara Bittolo, Erika Tissino, Filippo Vit, Elena Vendramini, Luca Laurenti, Giovanni D'Arena, Jacopo Olivieri, Gabriele Pozzato, Francesco Zaja, Annalisa Chiarenza, Francesco Di Raimondo, Antonella Zucchetto, Riccardo Bomben, Francesca Maria Rossi, Giovanni Del Poeta, Michele Dal Bo, and Valter Gattei Haematologica 2020 [Epub ahead of print] Citation: Federico Pozzo, Tamara Bittolo, Erika Tissino, Filippo Vit, Elena Vendramini, Luca Laurenti, Giovanni D'Arena, Jacopo Olivieri, Gabriele Pozzato, Francesco Zaja, Annalisa Chiarenza, Francesco Di Raimondo, Antonella Zucchetto, Riccardo Bomben, Francesca Maria Rossi, Giovanni Del Poeta, Michele Dal Bo, and Valter Gattei SF3B1-mutated chronic lymphocytic leukemia shows evidence of NOTCH1 pathway activation including CD20 downregulation. Haematologica. 2020; 105:xxx doi:10.3324/haematol.2020.261891 Publisher's Disclaimer. E-publishing ahead of print is increasingly important for the rapid dissemination of science. Haematologica is, therefore, E-publishing PDF files of an early version of manuscripts that have completed a regular peer review and have been accepted for publication. E-publishing of this PDF file has been approved by the authors. After having E-published Ahead of Print, manuscripts will then undergo technical and English editing, typesetting, proof correction and be presented for the authors' final approval; the final version of the manuscript will