Equine Stress
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Product List March 2019 - Page 1 of 53
Wessex has been sourcing and supplying active substances to medicine manufacturers since its incorporation in 1994. We supply from known, trusted partners working to full cGMP and with full regulatory support. Please contact us for details of the following products. Product CAS No. ( R)-2-Methyl-CBS-oxazaborolidine 112022-83-0 (-) (1R) Menthyl Chloroformate 14602-86-9 (+)-Sotalol Hydrochloride 959-24-0 (2R)-2-[(4-Ethyl-2, 3-dioxopiperazinyl) carbonylamino]-2-phenylacetic 63422-71-9 acid (2R)-2-[(4-Ethyl-2-3-dioxopiperazinyl) carbonylamino]-2-(4- 62893-24-7 hydroxyphenyl) acetic acid (r)-(+)-α-Lipoic Acid 1200-22-2 (S)-1-(2-Chloroacetyl) pyrrolidine-2-carbonitrile 207557-35-5 1,1'-Carbonyl diimidazole 530-62-1 1,3-Cyclohexanedione 504-02-9 1-[2-amino-1-(4-methoxyphenyl) ethyl] cyclohexanol acetate 839705-03-2 1-[2-Amino-1-(4-methoxyphenyl) ethyl] cyclohexanol Hydrochloride 130198-05-9 1-[Cyano-(4-methoxyphenyl) methyl] cyclohexanol 93413-76-4 1-Chloroethyl-4-nitrophenyl carbonate 101623-69-2 2-(2-Aminothiazol-4-yl) acetic acid Hydrochloride 66659-20-9 2-(4-Nitrophenyl)ethanamine Hydrochloride 29968-78-3 2,4 Dichlorobenzyl Alcohol (2,4 DCBA) 1777-82-8 2,6-Dichlorophenol 87-65-0 2.6 Diamino Pyridine 136-40-3 2-Aminoheptane Sulfate 6411-75-2 2-Ethylhexanoyl Chloride 760-67-8 2-Ethylhexyl Chloroformate 24468-13-1 2-Isopropyl-4-(N-methylaminomethyl) thiazole Hydrochloride 908591-25-3 4,4,4-Trifluoro-1-(4-methylphenyl)-1,3-butane dione 720-94-5 4,5,6,7-Tetrahydrothieno[3,2,c] pyridine Hydrochloride 28783-41-7 4-Chloro-N-methyl-piperidine 5570-77-4 -

Drug Screen Req. 06.2016.Docx Rev 2 Date 06-16 Page 1 of 2
TRUESDAIL LABORATORIES, INC. EXCELLENCE IN INDEPENDENT TESTING Established 1931 3337 MICHELSON DRIVE, SUITE CN 750 IRVINE, CALIFORNIA 92612 (714) 730-6239 FAX (714) 730-6462 www. truesdail.com DRUG SCREEN REQUEST FORM SAMPLE TYPE: Urine Blood Plasma Serum Collection Date: ______________ Doctor: ______________________________ Animal Name, HIP # or Other I.D.:______________________________________ Species: _________ Sex: ________ Owner’s name:_______________________ Pre-Purchase Screening (performed by LC/MS) LEVEL Ι: Includes only nonsteroidal anti-inflammatory drugs (phenylbutazone, $100.00 oxyphenbutazone, flunixin, naproxen, ketoprofen, firocoxib, diclofenac and meclofenamic acid). (Requires a minimum of 2 mL serum or plasma or 10mL of urine) LEVEL ΙΙ: Includes LEVEL Ι drugs plus testing for Domosedan (detomidine), $175.00 fluphenazine, acepromazine, promazine, chlorpromazine, triflupromazine, imipramine, propionylpromazine, clomipramine, and reserpine. (Requires a minimum of 4 mL of serum or plasma or 15mL of urine) LEVEL ΙΙΙ: Includes LEVEL Ι and ΙΙ drugs plus testing for butorphanol, triamcinolone $250.00 acetonide, betamethasone, dexamethasone, flumethasone, isoflupredone, predisone, methylprednisolone, prednisolone, albuterol, clenbuterol, terbutaline, and pirbuterol. (Requires a minimum of 5 mL of serum or plasma or 20mL of urine) LEVEL ΙV: TOBA Protocol Testing (urine and blood is required for this test) $300.00 (Requires a minimum of 6 mL of serum or plasma and 25mL of urine) *Rush testing is available for Level I, II or III -

Crystal Structure of Dopamine D1 Receptor in Complex with G Protein and a Non-Catechol Agonist
ARTICLE https://doi.org/10.1038/s41467-021-23519-9 OPEN Crystal structure of dopamine D1 receptor in complex with G protein and a non-catechol agonist Bingfa Sun 1,7, Dan Feng1,7, Matthew Ling-Hon Chu1, Inbar Fish1, Silvia Lovera2, Zara A. Sands2,6, Sebastian Kelm 3, Anne Valade2, Martyn Wood2, Tom Ceska 3, Tong Sun Kobilka1, Florence Lebon4 & ✉ Brian K. Kobilka 1,5 Dopamine D1 receptor (D1R) is an important drug target implicated in many psychiatric and 1234567890():,; neurological disorders. Selective agonism of D1R are sought to be the therapeutic strategy for these disorders. Most selective D1R agonists share a dopamine-like catechol moiety in their molecular structure, and their therapeutic potential is therefore limited by poor pharmaco- logical properties in vivo. Recently, a class of non-catechol D1R selective agonists with a distinct scaffold and pharmacological properties were reported. Here, we report the crystal structure of D1R in complex with stimulatory G protein (Gs) and a non-catechol agonist Compound 1 at 3.8 Å resolution. The structure reveals the ligand bound to D1R in an extended conformation, spanning from the orthosteric site to extracellular loop 2 (ECL2). Structural analysis reveals that the unique features of D1R ligand binding pocket explains the remarkable selectivity of this scaffold for D1R over other aminergic receptors, and sheds light on the mechanism for D1R activation by the non-catechol agonist. 1 ConfometRx, Inc., Santa Clara, CA, USA. 2 UCB Pharma, Braine-l’Alleud, Belgium. 3 UCB Pharma, Slough, UK. 4 UCB Pharma, Anderlecht, Belgium. 5 Department of Molecular and Cellular Physiology, Stanford University School of Medicine, Stanford, CA, USA. -

Detomidine and Butorphanol for Standing Sedation in a Range of Zoo-Kept Ungulate Species
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Ghent University Academic Bibliography Journal of Zoo and Wildlife Medicine 48(3): 616–626, 2017 Copyright 2017 by American Association of Zoo Veterinarians DETOMIDINE AND BUTORPHANOL FOR STANDING SEDATION IN A RANGE OF ZOO-KEPT UNGULATE SPECIES Tim Bouts, D.V.M., M.Sc., Dip. E.C.Z.M., Joanne Dodds, V.N., Karla Berry, V.N., Abdi Arif, M.V.Sc., Polly Taylor, Vet. M. B., Ph. D., Dip. E.C.V.A.A., Andrew Routh, B. V. Sc., Cert. Zoo. Med., and Frank Gasthuys, D.V.M., Ph. D., Dip. E.C.V.A.A. Abstract: General anesthesia poses risks for larger zoo species, like cardiorespiratory depression, myopathy, and hyperthermia. In ruminants, ruminal bloat and regurgitation of rumen contents with potential aspiration pneumonia are added risks. Thus, the use of sedation to perform minor procedures is justified in zoo animals. A combination of detomidine and butorphanol has been routinely used in domestic animals. This drug combination, administered by remote intramuscular injection, can also be applied for standing sedation in a range of zoo animals, allowing a number of minor procedures. The combination was successfully administered in five species of nondomesticated equids (Przewalski horse [Equus ferus przewalskii; n ¼ 1], onager [Equus hemionus onager; n ¼ 4], kiang [Equus kiang; n ¼ 3], Grevy’s zebra [Equus grevyi; n ¼ 4], and Somali wild ass [Equus africanus somaliensis; n ¼ 7]), with a mean dose range of 0.10–0.17 mg/kg detomidine and 0.07–0.13 mg/kg butorphanol; the white (Ceratotherium simum simum; n ¼ 12) and greater one-horned rhinoceros (Rhinoceros unicornis; n ¼ 4), with a mean dose of 0.015 mg/kg of both detomidine and butorphanol; and Asiatic elephant bulls (Elephas maximus; n ¼ 2), with a mean dose of 0.018 mg/kg of both detomidine and butorphanol. -

PRACTITIONER's GUIDE Perfect for Routine Procedures on Horses That DON't Want to Have Anything to DO with Routine Procedure
DORMOSEDAN GEL is a registered trademark of Orion Corporation and is distributed by Pfizer Inc. ©2010 Pfizer Inc. All rights reserved. DOR0710058 iMpORtANt SAfEty iNfORMAtiON DORMOSEDAN GEL® is contraindicated in horses with known hypersensitivity to detomidine. Intravenous potentiated sulfonamides should not be used in anesthetized or sedated horses, as potentially fatal dysrhythmias may occur. Do not use DORMOSEDAN GEL in horses with pre-existing atrio-ventricular (AV) or sino-atrial (SA) blocks, cardiovascular disease, respiratory disorders, liver or kidney diseases, or in conditions of shock, severe debilitation or stress due to extreme heat, cold, fatigue or high altitude. Appropriate precautions should be taken while handling and using gel dosing syringes, as DORMOSEDAN GEL can be absorbed following direct exposure to skin, eyes or mouth, and may cause irritation. The use of impermeable gloves is advised. Please see the full prescribing information, or go to www.DormosedanGel.com. www.DormosedanGel.com PerfEct for routine procedures on horses hERE’S hOw DORMOSEDAN GEL wORkS: DORMOSEDAN GEL cAN bE ADMiNiStERED by yOuR thAt don’t want tO hAvE anything tO do cLiENtS fOR A wiDE vARiEty Of routiNE procEDuRES. Detomidine is a potent and relatively selective α2-adrenoceptor agonist with a with routine procedures. central effect inhibiting the transmission of norepinephrine-mediated nervous Depending on the horse’s specific needs, DORMOSEDAN GEL® is impulses. In the animal, the level of consciousness is lowered. The well-characterized an effective standing sedation for use prior to stressful situations profile of detomidine at the receptor level explains its predictable, dose-dependent and minor, nonpainful husbandry procedures, including: pharmacological effects. -

The Effect of Dexmedetomidine and Clonidine on the Inflammatory Response in Critical Illness: a Systematic Review of Animal and Human Studies Charles A
Flanders et al. Critical Care (2019) 23:402 https://doi.org/10.1186/s13054-019-2690-4 RESEARCH Open Access The effect of dexmedetomidine and clonidine on the inflammatory response in critical illness: a systematic review of animal and human studies Charles A. Flanders1† , Alistair S. Rocke1†, Stuart A. Edwardson2, J. Kenneth Baillie1 and Timothy S. Walsh1,2,3* Abstract Background: The α2 agonists, dexmedetomidine and clonidine, are used as sedative drugs during critical illness. These drugs may have anti-inflammatory effects, which might be relevant to critical illness, but a systematic review of published literature has not been published. We reviewed animal and human studies relevant to critical illness to summarise the evidence for an anti-inflammatory effect from α2 agonists. Methods: We searched PubMed, the Cochrane library, and Medline. Animal and human studies published in English were included. Broad search terms were used: dexmedetomidine or clonidine, sepsis, and inflammation. Reference lists were screened for additional publications. Titles and abstracts were screened independently by two reviewers and full-text articles obtained for potentially eligible studies. Data extraction used a bespoke template given study diversity, and quality assessment was qualitative. Results: Study diversity meant meta-analysis was not feasible so descriptive synthesis was undertaken. We identified 30 animal studies (caecal ligation/puncture (9), lipopolysaccharide (14), acute lung injury (5), and ischaemia-reperfusion syndrome (5)), and 9 human studies. Most animal (26 dexmedetomidine, 4 clonidine) and all human studies used dexmedetomidine. In animal studies, α2 agonists reduced serum and/or tissue TNFα (20 studies), IL-6 (17 studies), IL-1β (7 studies), NFκB (6 studies), TLR4 (6 studies), and a range of other mediators. -

Travelers' Guideline for Prohibited And/Or Controlled Medicines and Drugs in the UAE and Quantities Permitted
This is an alphabetical list of INCB and MOH&P controlled Narcotics / Psychotropics / Controlled (CD) and Semi Controlled (SCD) Drugs used for medical purposes, their Scheduling and level of restrictions to carry with travllers to the UAE, with specific medical reasons and supporting documents . Allowed Quantity & Documents to be kept with SL # GENERIC NAME - (Alphabetical Order) CATEGORY (Control Level) the traveller 1 (+) – LYSERGIDE (LSD, LSD-25) Psychotropic Schedule I Prohibited Quantity for the period of stay or a maximum one 2 2c-B Psychotropic Schedule II month use whichever is less. Medical prescription and attested medical report is required 3 3-methylfentanyl NARCOTIC SCHEDULE – IV Prohibited 4 3-methylthiofentanyl NARCOTIC SCHEDULE – IV Prohibited 5 4 – Methylaminorex Psychotropic Schedule I Prohibited 6 4-MTA Psychotropic Schedule I Prohibited 7 Acetorphine NARCOTIC SCHEDULE – IV Prohibited 8 Acetyl-alpha-methylfentanyl NARCOTIC SCHEDULE – IV Prohibited Quantity for the period of stay or a maximum one 9 Acetyldihydrocodeine Narcotic Schedule II month use whichever is less. Medical prescription and attested medical report is required Quantity for the period of stay or a maximum one 10 Acetylmethadol NARCOTIC SCHEDULE – I month use whichever is less. Medical prescription and attested medical report is required Quantity for the period of stay or a maximum one 11 Agomelatine SCD month use whichever is less and need a Medical prescription. Quantity for the period of stay or a maximum one 12 Alfentanyl NARCOTIC SCHEDULE – I month use whichever is less. Medical prescription and attested medical report is required 1/31 This is an alphabetical list of INCB and MOH&P controlled Narcotics / Psychotropics / Controlled (CD) and Semi Controlled (SCD) Drugs used for medical purposes, their Scheduling and level of restrictions to carry with travllers to the UAE, with specific medical reasons and supporting documents . -
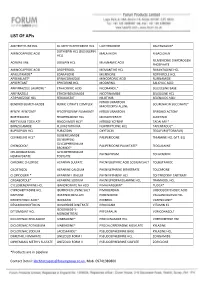
LIST of Apis
LIST OF APIs AMITRIPTYLINE HCL DL-METHYLEPHEDRINE HCL LIOTHYRONINE RALTEGRAVIR* DOTHIEPIN HCL (DOSULEPIN AMINOCAPROIC ACID MALATHION R-BACLOFEN HCL) RILMENIDINE DIHYDROGEN ACRIVASTINE DOXEPIN HCL MEFENAMIC ACID PHOSPHATE AMINOCAPROIC ACID DROPERIDOL MEMANTINE HCL RIMANTADINE HCL APALUTAMIDE* EDARAVONE MILRINONE ROPINIROLE HCL APREMILAST* EFINACONAZOLE MINODRONIC ACID RUFINAMIDE APREPITANT EPHEDRINE HCL MODAFINIL SALICYLIC ACID ARIPIPRAZOLE LAUROXIL* ETHACRYNIC ACID NICORANDIL* SELEGELINE BASE ARIPIRAZOLE ETHOXYBENZAMIDE NICOTINAMIDE SELEGILINE HCL ATIPAMEZOLE HCL FEBUXOSTAT NILOTINIB SELENIUOS ACID NITROFURANTOIN BENDROFLUMETHIAZIDE FERRIC CITRATE COMPLEX SOLIFENACIN SUCCINATE* MACROCRYSTALLINE BENZYL BENZOATE FESOTERODINE FUMARATE NITROFURANTOIN SPIRONOLACTONE BORTEZOMIB FEXOFENADINE HCL MONOHYDRATE SUNITINIB BRETYLIUM TOSYLATE FINGOLIMOD HCL* NITROGLYCERINE TADALAFIL* BRINZOLAMIDE FLUVASTATIN NA NORTRIPTLYINE HCL TAPENTADOL* BUPROPION HCL FURAZIDIN OXYTOCIN TEGAFUR (FTORAFUR) GLIBENCLAMIDE CEVIMELINE HCL* PALIPERIDONE THIAMINE HCL (VIT. B1) (GLYBURIDE) GLYCOPYRRONIUM CHENODIOL* PALIPERIDONE PALMITATE* TIOGUANINE BROMIDE* CHLOROBUTANOL GLYCOPYRRONIUM PHENAZEPAM TOLAZAMIDE HEMIHYDRATE TOSYLATE CHROMIC CHLORIDE HEPARAN SULFATE PHENYLBUTYRIC ACID SODIUM SALT TOLBUTAMIDE CILOSTAZOL HEPARINE CALCIUM PHENYLEPHRINE BITARTRATE TOLCAPONE CLOPIDOGREL* HEPARINE LITHIUM PHENYLEPHRINE HCL TOLTERODINE TARTRATE CRISABOROLE* HEPARINE SODIUM PHENYLPROPANOLAMINE HCL TRAMADOL HCL CYCLOBENZAPRINE HCL IBANDRONATE NA H2O PIMAVANSERIN* TUDCA* CYPROHEPTADINE -

Marrakesh Agreement Establishing the World Trade Organization
No. 31874 Multilateral Marrakesh Agreement establishing the World Trade Organ ization (with final act, annexes and protocol). Concluded at Marrakesh on 15 April 1994 Authentic texts: English, French and Spanish. Registered by the Director-General of the World Trade Organization, acting on behalf of the Parties, on 1 June 1995. Multilat ral Accord de Marrakech instituant l©Organisation mondiale du commerce (avec acte final, annexes et protocole). Conclu Marrakech le 15 avril 1994 Textes authentiques : anglais, français et espagnol. Enregistré par le Directeur général de l'Organisation mondiale du com merce, agissant au nom des Parties, le 1er juin 1995. Vol. 1867, 1-31874 4_________United Nations — Treaty Series • Nations Unies — Recueil des Traités 1995 Table of contents Table des matières Indice [Volume 1867] FINAL ACT EMBODYING THE RESULTS OF THE URUGUAY ROUND OF MULTILATERAL TRADE NEGOTIATIONS ACTE FINAL REPRENANT LES RESULTATS DES NEGOCIATIONS COMMERCIALES MULTILATERALES DU CYCLE D©URUGUAY ACTA FINAL EN QUE SE INCORPOR N LOS RESULTADOS DE LA RONDA URUGUAY DE NEGOCIACIONES COMERCIALES MULTILATERALES SIGNATURES - SIGNATURES - FIRMAS MINISTERIAL DECISIONS, DECLARATIONS AND UNDERSTANDING DECISIONS, DECLARATIONS ET MEMORANDUM D©ACCORD MINISTERIELS DECISIONES, DECLARACIONES Y ENTEND MIENTO MINISTERIALES MARRAKESH AGREEMENT ESTABLISHING THE WORLD TRADE ORGANIZATION ACCORD DE MARRAKECH INSTITUANT L©ORGANISATION MONDIALE DU COMMERCE ACUERDO DE MARRAKECH POR EL QUE SE ESTABLECE LA ORGANIZACI N MUND1AL DEL COMERCIO ANNEX 1 ANNEXE 1 ANEXO 1 ANNEX -

Drug Delivery System for Use in the Treatment Or Diagnosis of Neurological Disorders
(19) TZZ __T (11) EP 2 774 991 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 10.09.2014 Bulletin 2014/37 C12N 15/86 (2006.01) A61K 48/00 (2006.01) (21) Application number: 13001491.3 (22) Date of filing: 22.03.2013 (84) Designated Contracting States: • Manninga, Heiko AL AT BE BG CH CY CZ DE DK EE ES FI FR GB 37073 Göttingen (DE) GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO •Götzke,Armin PL PT RO RS SE SI SK SM TR 97070 Würzburg (DE) Designated Extension States: • Glassmann, Alexander BA ME 50999 Köln (DE) (30) Priority: 06.03.2013 PCT/EP2013/000656 (74) Representative: von Renesse, Dorothea et al König-Szynka-Tilmann-von Renesse (71) Applicant: Life Science Inkubator Betriebs GmbH Patentanwälte Partnerschaft mbB & Co. KG Postfach 11 09 46 53175 Bonn (DE) 40509 Düsseldorf (DE) (72) Inventors: • Demina, Victoria 53175 Bonn (DE) (54) Drug delivery system for use in the treatment or diagnosis of neurological disorders (57) The invention relates to VLP derived from poly- ment or diagnosis of a neurological disease, in particular oma virus loaded with a drug (cargo) as a drug delivery multiple sclerosis, Parkinsons’s disease or Alzheimer’s system for transporting said drug into the CNS for treat- disease. EP 2 774 991 A1 Printed by Jouve, 75001 PARIS (FR) EP 2 774 991 A1 Description FIELD OF THE INVENTION 5 [0001] The invention relates to the use of virus like particles (VLP) of the type of human polyoma virus for use as drug delivery system for the treatment or diagnosis of neurological disorders. -

Effective Standing Sedation and Analgesia
PF2912 Dormosedan flyer v4 11/29/06 3:30 PM Page 1 Effective Standing Sedation and Analgesia Dormosedan® (detomidine hydrochloride) from Pfizer Animal Health – Provide the best for your patients and your clients today’s equine veterinary practices, standing sedation is a critical Dr. Henry has used Dormosedan as a tool to help expedite dental proce- part of many key procedures done on-farm and in the referral clinic. dures – an area he has found to be of great benefit to the health of his When choosing between the molecules available, veterinarians clients’ horses and to his entire practice. In must consider safety, cost effectiveness for the client and the “Dentistry is a scheduled visit that can be made in advance,” said Dr. practice, and – above all – the ultimate efficacy of the product in achieving Henry. “It can contribute to the bottom line of the practice due to the fact an appropriate level of sedation for the needed procedure. that all of the horses in the practice should have dental care once a year. Not to mention that dentistry can be scheduled when the veterinarian has Sedation and Analgesia more time to devote to the procedure – such as fall or winter.” In charging clients for the sedation and analgesia portion of their hors- Sedation may be the practitioners’ first consideration in standing proce- es’ dentistry needs, Dr. Henry finds that charging for “dental sedation” dures such as dental care, suturing wounds and castrations, but choosing helps to get clients thinking about the fact that this type of sedation is a molecule that provides a proven level of analgesia offers a more humane different than that given for shorter procedures, such as joint injections. -

The Protective and Hemodynamic Effects of Dexmedetomidine on Hypertensive Cerebral Hemorrhage Patients in the Perioperative Period
EXPERIMENTAL AND THERAPEUTIC MEDICINE 12: 2903-2908, 2016 The protective and hemodynamic effects of dexmedetomidine on hypertensive cerebral hemorrhage patients in the perioperative period JUNHUI ZHAO1* and CHUIXIAN ZHOU2* 1Department of Anesthesiology, Weifang Medical University, Weifang, Shandong 261053; 2Department of Neurosurgery, Weifang People's Hospital, Weifang, Shandong 261041, P.R. China Received March 2, 2016; Accepted September 13, 2016 DOI: 10.3892/etm.2016.3711 Abstract. The aim of the present study was to analyze the operation (T3) were higher than the basal level (T1) in the protective and hemodynamic effects of dexmedetomidine midazolam group, but close to the basal level in the dexme- in hypertensive cerebral hemorrhage (HCH) patients during detomidine group (P<0.05). The serum concentration of NSE perioperative period. In total, 50 HCH patients were selected and S100β in the two groups showed no difference (P>0.05) and randomly divided into two groups, one group was at the end of operation (T5), but after 24 h of operation (T7) administered with dexmedetomidine and the other groups NSE and S100β in the dexmedetomidine group were signifi- with midazolam. The mean arterial pressure (MAP), heart cantly lower compared to the midazolam group (P<0.05). rate (HR) and blood oxygen saturation (SpO2) were moni- In conclusion, the administration of dexmedetomidine for tored in the two groups of patients before and during the patients with HCH during perioperative period is safe and operation. The MAP, HR, SpO2 and PETCO2 recorded 5 min serves as an effective sedative, without causing respiratory after admission into the operation room was considered T1, depression and does not influence stable haemodynamics the same parameters recorded 10 min after drug adminis- with certain brain protective effect.