The Pathology Workforce and Clinical Licensure
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

VIROLOGY for MEDICAL LABORATORY STUDENTS Mwamisi, Muthwii, Mwala, Mokua
VIROLOGY for MEDICAL LABORATORY STUDENTS Mwamisi, Muthwii, Mwala, Mokua To the Reader Virology is an important subject in Medical Laboratory Sciences. Medical Laboratory Medicine puts a technologist/technician in the fore front in diagnosis and proper interpretation of the correct diseases by a clinician. Research on viruses, therefore, has gained a lot of momentum in recent years due to the Medical importance they contribute to the field of medicine. A lot of this work goes through the medical laboratory Department where it is handled by the medical laboratory students, especially, on practical experience. The purpose of this manual is, therefore, to provide to the student adequate up-to-date information in preparation for the work that the student will perform in any Medical Laboratory Department, whether research or routine work. We hope this information will not only be useful to the medical laboratory Students or staff, but!also will be a reference material for others in Health or health related field. Joseph M. Mwamisi, PhD Samson M. Muthwii PhD Denis M. Mwala M.Se. John M. Mokua M.Se., ) 2 TABLE OF CONTENTS 1. VIROLOGY 6 1.1 Definition of Virology 6 1.2 Definition of Viruses 6 1.3 Differences between Bacteria and Viruses 7 1.4 Morphology of Viruses 7 2. CLASSIFICATION OF VIRUSES 9 2.1 Classification 9 Epidemiological Analysis 9 Physical and Chemical Properties Analysis 9 DNA Viruses 9 RNA Viruses 9 Enteric Viruses 10 Respiratory Viruses ' 10 Arthropod-Borne Viruses (Arboviruses) 10 2.2. DNA Viruses 10 Poxvirusae 10 Ridoviridae 10 2.3. RNA Viruses 10 Picornaviridae 11 3. -

Medical Laboratory Scientist LIMITED ENTRY BACHELOR of APPLIED SCIENCE (BAS) REQUIRED CREDITS: 120 DEGREE CODE: MLS-BAS
MEDICAL LABORATORY PROGRAM Medical Laboratory Scientist LIMITED ENTRY BACHELOR OF APPLIED SCIENCE (BAS) REQUIRED CREDITS: 120 DEGREE CODE: MLS-BAS This is a limited-entry program. Some of these courses are program prerequisites and MUST be completed before a student is considered eligible for entry into the Program. Students MUST attend a Health Programs orientation and meet with a Health Programs advisor for additional advisement. DESCRIPTION The Medical Laboratory Scientist (MLS) is an important member of the health care team in hospitals, clinics, medical research and teaching centers, and is an indispensable participant with physicians in providing critical diagnostic information. The MLS functions as a dependable, ambitious and highly motivated professional capable of handling high stress situations with ease and confidence. The Medical Laboratory Scientist performs and interprets diagnostic laboratory procedures using state-of-the-art instrumentation to aid in the detection, diagnosis, and treatment of disease; monitors the standards of accuracy and precision in the performance of tests; performs routine maintenance; analyzes and corrects instrument problems; researches, evaluates, and implements new procedures; and may be responsible for fiscal/personnel management of laboratory. The Bachelor of Applied Science degree in Medical Laboratory Scientist combines academic and laboratory courses on campus with practical experience at clinical affiliate sites. The program is accredited by the National Accrediting Agency for Clinical Laboratory Sciences (NAACLS), 5600 N. River Road, Suite 720, Rosemont, IL 60018-5119, (877) 939-3597. Students successfully completing the program are eligible to take a national certifying examination. STUDENT LEARNING OUTCOMES • Select appropriate courses of action in accordance with established laboratory procedures. -

Medical Laboratory Assistant, a Suggested Guide for A
p .7/ tWI .7- y,1.. P O R T R ESUM ED 013 321 VT 002 2L4 MEDICAL LABORATORY ASSISTANT, A SUGGESTEDGUIDE FOR A TRAINING PROGRAM. OFFICE OF EDUCATION, WASHINGTON, D.C. REPORT NUMBER 0E-87017 PUB DATE 66 EDRS PRICE MF-$0.50 HC-$4.92 123P. DESCRIPTORS-. *MEDICAL LABORATORY ASSISTANTS,TEACHING GUIDES, *PROGRAM PLANNING, PROGRAM DEVELOPMENT,*CURRICULUM GUIDES, CURRICULUM, *HEALTH OCCUPATIONSEDUCATION, POST SECONDARY EDUCATION, MDTA PROGRAMS, INFORMATION IS GIVEN TO ASSIST IN ORGANIZINGAND ADMINISTERING A TRAINING PROGRAM FOR MEDICAL LABORATORY ASSISTANTS IN A VARIETY OF SETTINGS AND TO PROVIDEGUIDANCE IN ESTABLISHING NEW PROGRAMS AND IN EVALUATINGEXISTING ONES. THE MATERIAL WAS PREPARED UNDER THE DIRECTIONOF THE NATIONAL COMMITTEE =OR CAREERS IN MEDICAL TECHNOLOGY.PATHOLOGISTS AND MEDICAL TECHNOLOGISTS PARTICIPATEDIN THE ORGANIZATIONAL AND DEVELOPMENTAL STAGES. ALL MATERIAL WAS REVIEWEDBY A REPRESENTATIVE NATIONAL GROUP OF EXPERTCONSULTANTS IN THE FIELD OF LABORATORY MEDICINE. THE 12-MONTH PROGRAM WAS DESIGNED FOR HIGH SCHOOL GRADUATES OR THEIREQUIVALENT TO SE ADMINISTERED BY A TEACHING STAFF COMPOSEDOF A NATIONAL DIRECTOR, A. TEACHING SUPERVISOR, ANDINSTRUCTORS. AN OUTLINE OF INFORMATIONAL MATERIAL TO BE PRESENTEDIN THE CLASSROOM, LABORATORY PROCEDURES TO BE DEMONSTRATEDAND THEN PERFORMED AS DIRECT EXERCISES BY THE STUDENTS, AS WELLAS RELEVANT BIBLIOGRAPHIES, AUDIOVISUAL AIDS, AND STUDYQUESTIONS ARE PRESENTED FOR THE FOLLOWING UNITS (1) ORIENTATION TO THE CLINICAL LABORATORY,(2) BACTERIOLOGY,(3) SEROLOGY, (4) PARASITOLOGY,(5) HEMATOLOGY, (6) CLINICAL CHEMISTRY,(7) BLOOD BANKING,(8) ROUTINE ANALYSIS, AND (9) BASAL METABOLISM -- ELECTROCARDIOGRAPHY. THIS DOCUMENT IS AVAILABLE AS GPO NUMBER FS 5.267-07017 FOR 60 CENTS FROMSUPERINTENDENT OF DOCUMENTS, U.S. GOVERNMENT PRINTING OFFICE,WASHINGTON, D.C. 20402. (PS) cir 02281"2=10. -
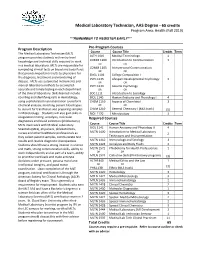
Medical Laboratory Technician 65 Credit AAS Program Guide
Medical Laboratory Technician, AAS Degree - 65 credits Program Area: Health (Fall 2019) ***REMEMBER TO REGISTER EARLY*** Program Description Pre-Program Courses Course Course Title Credits Term The Medical Laboratory Technician (MLT) program provides students with entry level ALTH 1410 Medical Terminology 1 knowledge and technical skills required to work COMM 1100 Introduction to Communication 3 in a medical laboratory. MLTs are responsible for OR OR COMM 1105 Interpersonal Communication completing clinical tests on blood and body fluids OR OR that provide important results to physicians for ENGL 1106 College Composition I the diagnosis, treatment and monitoring of PSYC 1135 Lifespan Developmental Psychology 3 disease. MLTs use automated instruments and OR OR manual laboratory methods to accomplish PSYC 1120 General Psychology accurate and timely testing in each department OR OR of the clinical laboratory. Skills learned include SOC 1111 Introduction to Sociology counting and identifying cells in Hematology, BIOL 1140 Human Anatomy and Physiology I 4 using sophisticated instrumentation to perform CHEM 1110 Aspects of Chemistry I 3 chemical analysis, matching patient blood types OR OR to donors for transfusion and preparing samples CHEM 1210 General Chemistry I (MLS track) (5) in Microbiology. Students will also gain skills in BIOL 1170 Microbiology 3 coagulation testing, urinalysis, molecular Required Courses diagnostics and blood collection (phlebotomy). MLTs must work with Medical Laboratory Course Course Title Credits Term Scientists (MLS), physicians, phlebotomists, BIOL 1141 Human Anatomy and Physiology II 4 MLTN 1400 Introduction to Medical Laboratory nurses and other healthcare professionals as 2 they collect patient samples, communicate test Techniques and Instrumentation results and resolve diagnostic discrepancies. -

Laboratory Medicine: a National Status Report
Laboratory Medicine: A National Status Report Prepared for: Division of Laboratory Systems National Center for Preparedness, Detection, and Control of Infectious Diseases Centers for Disease Control and Prevention Prepared by: The Lewin Group Under subcontract to Battelle Memorial Institute May 2008 Laboratory Medicine: A National Status Report Prepared for: Division of Laboratory Systems National Center for Preparedness, Detection, and Control of Infectious Diseases Centers for Disease Control and Prevention Prepared by: The Lewin Group Julie Wolcott, MA Amanda Schwartz Clifford Goodman, PhD May 2008 Laboratory Medicine: A National Status Report Executive Summary TABLE OF CONTENTS ACKNOWLEDGMENTS v EXECUTIVE SUMMARY 1 The Value of Laboratory Medicine to Health Care ....................................................................... 2 Market Profile of the Laboratory Medicine Sector ........................................................................ 3 Laboratory Medicine Workforce...................................................................................................... 4 Quality and the Total Testing Process............................................................................................. 4 Quality Systems and Performance Measurement ......................................................................... 6 Laboratory Information Systems ..................................................................................................... 6 Federal Regulatory Oversight Of Laboratory Medicine.............................................................. -

Medical Laboratory Science Completion
College of Science & Health Professions 2020-2021 Medical Laboratory Science: 3+1 Pathway First Year – Freshman Fall Semester Spring Semester *ENGL 1113 Freshman Composition I *ENGL 1213 Freshman Composition II *BIOL 1013/1011 Introductory Biology I and Lab CHEM 1123/1131 General Chemistry I and Lab *UNIV 1003 University Strategies *POLS 1113 American Federal Government *HIST 1483 or 1493 American History 1876 **Humanities (3 hrs) *MATH 1513 College Algebra *H ED 1113 Personal Health or NUTR 1653 Basic Nutrition Total Hours 16 Total Hours 16 Second Year – Sophomore Fall Semester Spring Semester BIOL 1023/1021 Introductory Biology II and Lab BIOL 2224 Microbiology CHEM 1223/1231 General Chemistry II and Lab CHEM 3123/3132 Organic Chemistry I and Lab **Communications (3 hrs) *PSYC 1113 Introduction to Psychology *CS 1003 Computers in Modern Society **Global Perspective (3 hrs) 3 hrs free electives Total Hours 17 Total Hours 15 Third Year – Junior Fall Semester Spring Semester BIOL 3314 Human Physiology BIOL 4354 Immunology BIOL 3114 Genetics CHEM 4214 Biochemistry **Humanities (3 hrs) ***3 – 4 hrs major electives 4 hrs free electives 4 hrs free electives Total Hours 15 Total Hours 15 Fourth Year – Senior (Internship at School of Medical Technology) Summer Semester Fall Semester Spring Semester MEDT 4351 Topics in Med Tech MEDT 4117 Clinical Microbiology MEDT 4216 Clinical Immunology MEDT 4325 Clinical Chemistry II MEDT 4125 Clinical Chemistry I MEDT 4236 Clinical Hematology Total Hours 6 Total Hours 12 Total Hours 12 Total Degree Plan Hours 124 *General Education Requirement. **See current catalog “General Requirements” for selection. ***Choose from an approved BIOL course. -

Medical Laboratory Technician Student Handbook
MEDICAL LABORATORY TECHNICIAN PROGRAM Student Handbook 2021-2022 Table of Contents Mission and Goals ......................................................................................................................................... 4 Program Description ............................................................................................................................. 4 Mission Statement ................................................................................................................................ 5 Program Outcomes ............................................................................................................................... 5 Program Goals ....................................................................................................................................... 5 Accreditation, Certification, Competencies, and Standards ......................................................................... 7 Accreditation ......................................................................................................................................... 7 Certification ........................................................................................................................................... 7 Entry Level Competencies ..................................................................................................................... 7 External Standards ............................................................................................................................... -

2020 - 2021 21250 Stevens Creek Blvd
Academic Year Medical Laboratory Technology 2020 - 2021 21250 Stevens Creek Blvd. Cupertino, CA 95014 408-864-5678 www.deanza.edu Biological, Health and Find your counselor at Environmental Sciences Division deanza.edu/our-counselors Kirsch Center, Room 228 408-864-8773 Please visit the Counseling and Advising Center to apply for degrees and for Clinical Laboratory Assistant academic planning assistance. Certificate of Achievement Certificate of Achievement Requirements The Clinical Laboratory Assistant Certificate of Achievement • Completion of all major courses with a C grade or higher. is available to students who have successfully completed the four required courses of study. The Certificate of Achievement Note: A maximum of six quarter units may be transferred from other academic institutions. is a 12-month course of study that prepares students for a career as a Clinical Laboratory Assistant through classroom Certificate of Achievement-Advanced Requirements study. Graduates of the certificate program have a recognized • Completion of all major courses with a C grade or higher. skill set for jobs as a laboratory assistant and opportunity for • Demonstrated proficiency in mathematics and English as advancement. evidenced by eligibility for MATH 114 and eligibility for any of the following: EWRT 1A, EWRT 1AH, EWRT 1AS with EWRT 1AT, Program Learning Outcomes: Upon completion, students will or ESL 5. be able to Note: A maximum of 18 quarter units may be transferred from other • Qualify and be eligible for jobs as a clinical laboratory academic institutions. assistant A.A./A.S. Degree Requirements 1. Completion of all General Education (GE) requirements 1. Meet the requirements for this certificate level. -

Medical Laboratory Science (Ms)
LOYOLA UNIVERSITY CHICAGO PARKINSON SCHOOL OF HEALTH SCIENCES AND PUBLIC HEALTH GRADUATE AND PROFESSIONAL ENROLLMENT MANAGEMENT • P 312.915.8950 • E [email protected] • W gpem.LUC.edu/apply MASTER OF SCIENCE IN MEDICAL LABORATORY SCIENCE (MS) The Master’s in Medical Laboratory Science (MS-MLS) program prepares students to become competent and qualified Medical Laboratory Scientists. The MS-MLS program focuses on training students to enhance the health of individuals, communities, and the larger global environment through the discovery, application, and dissemination of knowledge; and service with others. The program imparts exceptional leadership training, acquisition of a clinically relevant base of scientific knowledge, and critical thinking skills necessary to engage in clinical laboratory practice, industry, or research. The MS in Medical Laboratory Science prepares students to be competent in the following areas: • Laboratory • Hematology • Microbiology • Molecular Leadership • Immunohematology • Hemostasis Diagnostics • Clinical Chemistry • Immunology PROGRAM GOALS • Provide students with the competency and depth of knowledge in major disciplines of the clinical laboratory to enter the profession of medical laboratory science. • Facilitate the practice of strong written and verbal communication skills sufficient to serve the needs of patients, the health care team, scientific peers, and the public. • Cultivate the next generation of scientists’ leadership abilities to help guide the changing landscape of health care. PROGRAM STRUCTURE This is a full-time degree designed to be completed in 5 continuous semesters, including one summer session. The first three semesters students are based at Health Science Campus of Loyola University Chicago and consist of didactic lectures and student laboratory training experiences. The fourth and fifth semesters are largely composed of a clinical practicum, where students are immerged in laboratory medicine at one of our clinical affiliates. -

Medical Laboratory Technologist (MLT)
Medical Laboratory Technologist (MLT) Medical Laboratory Technologists in Foreign qualification recognition is the process of verifying New Brunswick that the knowledge, skills, work experience and education obtained in another country are comparable to the standards In New Brunswick, medical laboratory established for Canadian professionals and tradespersons. technology is a regulated profession. To work in this field, you are required to complete the foreign qualification Laboratory science involves How do I get my recognition process and provide all critical thinking, problem-solving, necessary documentation. analyzing specimens and employing credentials recognized? sophisticated equipment and You can start your application There is a need for MLTs in both big computer interfaces to ensure the abroad; however, you have to write cities and smaller communities across timely and accurate delivery of test the certification exam in Canada. New Brunswick; you can also find jobs results. It involves interpretation of The New Brunswick Society of in one of the two official languages of results and the relationship between Medical Laboratory Technologists the province: English or French. results and various medical conditions. (NBSMLT) (www.nbsmlt.nb.ca) is the regulatory agency in the province. Most MLTs work in hospitals, but The foreign credential recognition jobs are also available in private process is centralized, and it is done laboratories, community health clinics, The New Brunswick Society by the Canadian Society for Medical Public Health facilities, university of Medical Laboratory Laboratory Science (CSMLS) (www. research laboratories, biotechnology Technologists (NBSMLT) csmls.org). Please visit the CSML companies and specialty laboratories. was founded in 1948 when website and click on Internationally the New Brunswick branch Educated MLTs for more information To obtain a licence to practise in about specific requirements, costs and New Brunswick, an MLT must be of the Canadian Society of other resources. -

Model Job Description: Consultant Medical Virologist
Model job description: consultant medical virologist Title of employing body Title of post Appointment State whether the post is full or part time and state the number of programmed activities (PAs). State whether the post is a new or replacement post. State whether the appointee is expected to have a special interest, or is expected to develop such an interest to complement other consultants. If part of a managed network, give relevant information. Any applicant who is unable, for personal reasons, to work full time will be eligible to be considered for the post. If such a person is appointed, modification of the job content will be discussed on a personal basis with the employing body in consultation with consultant colleagues. General information Describe the location: city/town and surrounding area, size of population, etc. The employing body Give a detailed description of the hospital(s) served and its/their work, including details of the clinical specialties, whether or not there is an accident and emergency service, details of surgical, medical, paediatric, obstetrics, gynaecology, oncology units, organ transplant, bone marrow transplant, HIV, hepatitis and antenatal etc. and any planned developments. Describe hospital location, number of beds, including SCBU, PICU, ITU and HDU beds and range of clinical services, any planned changes or major developments, special features, management arrangements, etc. Give an outline description of the pathology departments and their relationship with each other and the rest of the hospital. If relevant, describe the relationship with university/medical school departments and research units. This should include any planned or proposed changes in the provision of pathology services. -

Medical Laboratory Immunology Programs
Medical Laboratory Immunology Programs: Rosalind Franklin University of Medicine and Science Chicago Area Clinical Immunology Training Program North Chicago, IL Program Director: Kenneth Beaman [email protected], [email protected] The training provides the fellow a unique opportunity to learn the skills, knowledge and experience necessary to become a successful medical laboratory immunologist from three different perspectives. The training program involves a series of rotations through the various laboratory sections at the Clinical Immunology Laboratory of the Rosalind Franklin University of Medicine and Science, Department of Pathology, Evanston Hospital and Children's Memorial Hospital, Department of Medicine, Clinical Immunology Laboratory. In addition to the rotations through the particular laboratories, courses in Laboratory Administration and Clinical Medicine will be included. More information can be found on the program website. University of North Carolina Hospitals, Immunology Clinical Microbiology/Immunology Laboratory UNC Hospitals, Chapel Hill, NC Program Director: John Schmitz, [email protected] The University of North Carolina Hospital's program has recently been revised to a three-year fellowship to prepare trainees in the field of laboratory immunology and clinical histocompatibility testing. Fellows receive training in all sections of the Clinical Immunology Laboratories (Infectious Disease, Autoimmune Disease, and Allergy Testing, Flow Cytometry and Molecular Immunology). In addition, fellows now receive training in Clinical Histocompatibility testing (HLA typing and antibody testing in support of solid organ and bone marrow transplant programs). Frequent interaction with physicians during rounds, clinical conferences, and consultations contributes extensively to the in depth of training provided. Fellows also receive laboratory management training and have the opportunity to participate in clinical and translational research with infectious disease, transplant and pathology faculty.