ROSITA Project
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Properties and Units in Clinical Pharmacology and Toxicology
Pure Appl. Chem., Vol. 72, No. 3, pp. 479–552, 2000. © 2000 IUPAC INTERNATIONAL FEDERATION OF CLINICAL CHEMISTRY AND LABORATORY MEDICINE SCIENTIFIC DIVISION COMMITTEE ON NOMENCLATURE, PROPERTIES, AND UNITS (C-NPU)# and INTERNATIONAL UNION OF PURE AND APPLIED CHEMISTRY CHEMISTRY AND HUMAN HEALTH DIVISION CLINICAL CHEMISTRY SECTION COMMISSION ON NOMENCLATURE, PROPERTIES, AND UNITS (C-NPU)§ PROPERTIES AND UNITS IN THE CLINICAL LABORATORY SCIENCES PART XII. PROPERTIES AND UNITS IN CLINICAL PHARMACOLOGY AND TOXICOLOGY (Technical Report) (IFCC–IUPAC 1999) Prepared for publication by HENRIK OLESEN1, DAVID COWAN2, RAFAEL DE LA TORRE3 , IVAN BRUUNSHUUS1, MORTEN ROHDE1, and DESMOND KENNY4 1Office of Laboratory Informatics, Copenhagen University Hospital (Rigshospitalet), Copenhagen, Denmark; 2Drug Control Centre, London University, King’s College, London, UK; 3IMIM, Dr. Aiguader 80, Barcelona, Spain; 4Dept. of Clinical Biochemistry, Our Lady’s Hospital for Sick Children, Crumlin, Dublin 12, Ireland #§The combined Memberships of the Committee and the Commission (C-NPU) during the preparation of this report (1994–1996) were as follows: Chairman: H. Olesen (Denmark, 1989–1995); D. Kenny (Ireland, 1996); Members: X. Fuentes-Arderiu (Spain, 1991–1997); J. G. Hill (Canada, 1987–1997); D. Kenny (Ireland, 1994–1997); H. Olesen (Denmark, 1985–1995); P. L. Storring (UK, 1989–1995); P. Soares de Araujo (Brazil, 1994–1997); R. Dybkær (Denmark, 1996–1997); C. McDonald (USA, 1996–1997). Please forward comments to: H. Olesen, Office of Laboratory Informatics 76-6-1, Copenhagen University Hospital (Rigshospitalet), 9 Blegdamsvej, DK-2100 Copenhagen, Denmark. E-mail: [email protected] Republication or reproduction of this report or its storage and/or dissemination by electronic means is permitted without the need for formal IUPAC permission on condition that an acknowledgment, with full reference to the source, along with use of the copyright symbol ©, the name IUPAC, and the year of publication, are prominently visible. -

FDA Approved Drugs with Broad Anti-Coronaviral Activity Inhibit SARS-Cov-2 in Vitro
bioRxiv preprint doi: https://doi.org/10.1101/2020.03.25.008482; this version posted March 27, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. FDA approved drugs with broad anti-coronaviral activity inhibit SARS-CoV-2 in vitro Stuart Weston1, Rob Haupt1, James Logue1, Krystal Matthews1 and Matthew B. Frieman*1 1 - Department of Microbiology and Immunology, University of Maryland School of Medicine, 685 W. Baltimore St., Room 380, Baltimore, MD, 21201, USA *Corresponding author. Email: [email protected] Key words: SARS-CoV-2, nCoV-2019, COVID-19, drug repurposing, FDA approved drugs, antiviral therapeutics, pandemic, chloroquine, hydroxychloroquine AbstraCt SARS-CoV-2 emerged in China at the end of 2019 and has rapidly become a pandemic with over 400,000 recorded COVID-19 cases and greater than 19,000 recorded deaths by March 24th, 2020 (www.WHO.org) (1). There are no FDA approved antivirals or vaccines for any coronavirus, including SARS-CoV-2 (2). Current treatments for COVID-19 are limited to supportive therapies and off-label use of FDA approved drugs (3). Rapid development and human testing of potential antivirals is greatly needed. A potentially quicker way to test compounds with antiviral activity is through drug re-purposing (2, 4). Numerous drugs are already approved for use in humans and subsequently there is a good understanding of their safety profiles and potential side effects, making them easier to test in COVID-19 patients. -

Designing Inhibitors Via Molecular Modelling Methods for Monoamine Oxidase Isozymes a and B Filiz Varnali Kadir Has Universit
DESIGNING INHIBITORS VIA MOLECULAR MODELLING METHODS FOR MONOAMINE OXIDASE ISOZYMES A AND B FİLİZ VARNALI KADİR HAS UNIVERSITY 2012 DESIGNING INHIBITORS VIA MOLECULAR MODELLING METHODS FOR MONOAMINE OXIDASE ISOZYMES A AND B FİLİZ VARNALI M.S. in Computational Biology and Bioinformatics, Kadir Has University, 2012 Submitted to the Graduate School of Science and Engineering in partial fulfilment of the requirements for the degree of Master of Science in Computational Biology and Bioinformatics KADİR HAS UNIVERSITY 2012 DESIGNING INHIBITORS VIA MOLECULAR MODELING METHODS FOR MONOAMINE OXIDASE ISOZYMES A AND B Abstract In drug development studies, a large number of new drug candidates (leads) have to be synthesized and optimized by changing several moieties of the leads in order to increase efficacies and decrease toxicities. Each synthesis of these new drug candidates include multi-steps procedures. Overall, discovering a new drug is a very time-consuming and very costly works. The development of molecular modelling programs and their applications in pharmaceutical research have been formalized as a field of study known computer assisted drug design (CADD) or computer assisted molecular design (CAMD). In this study, using the above techniques, Monoamine Oxidase isozymes, which play an essential role in the oxidative deamination of the biogenic amines, were studied. Compounds that inhibit these isozymes were shown to have therapeutic value in a variety of conditions including several psychiatric and neurological as well as neurodegenerative diseases. First, a series of new pyrazoline derivatives were screened using molecular modelling and docking methods and promising lead compounds were selected, and proposed for synthesis as novel selective MAO-A or –B inhibitors. -

The Effects of Antipsychotic Treatment on Metabolic Function: a Systematic Review and Network Meta-Analysis
The effects of antipsychotic treatment on metabolic function: a systematic review and network meta-analysis Toby Pillinger, Robert McCutcheon, Luke Vano, Katherine Beck, Guy Hindley, Atheeshaan Arumuham, Yuya Mizuno, Sridhar Natesan, Orestis Efthimiou, Andrea Cipriani, Oliver Howes ****PROTOCOL**** Review questions 1. What is the magnitude of metabolic dysregulation (defined as alterations in fasting glucose, total cholesterol, low density lipoprotein (LDL) cholesterol, high density lipoprotein (HDL) cholesterol, and triglyceride levels) and alterations in body weight and body mass index associated with short-term (‘acute’) antipsychotic treatment in individuals with schizophrenia? 2. Does baseline physiology (e.g. body weight) and demographics (e.g. age) of patients predict magnitude of antipsychotic-associated metabolic dysregulation? 3. Are alterations in metabolic parameters over time associated with alterations in degree of psychopathology? 1 Searches We plan to search EMBASE, PsycINFO, and MEDLINE from inception using the following terms: 1 (Acepromazine or Acetophenazine or Amisulpride or Aripiprazole or Asenapine or Benperidol or Blonanserin or Bromperidol or Butaperazine or Carpipramine or Chlorproethazine or Chlorpromazine or Chlorprothixene or Clocapramine or Clopenthixol or Clopentixol or Clothiapine or Clotiapine or Clozapine or Cyamemazine or Cyamepromazine or Dixyrazine or Droperidol or Fluanisone or Flupehenazine or Flupenthixol or Flupentixol or Fluphenazine or Fluspirilen or Fluspirilene or Haloperidol or Iloperidone -

33-Adrenoceptor-Mediated Relaxation Induced by Isoprenaline And
J. Smooth Muscle Res. 33 : 99-106. 99 The )32 and [33-Adrenoceptor-Mediated Relaxation Induced by Isoprenaline and Salbutamol in Guinea Pig Taenia Caecum Katsuo KOIKE, Tsukasa IcHiNo, Takahiro HORINOUCHI and Issei TAKAYANAGI Departmentof Chemical Pharmacology, Toho University School of PharmaceuticalSciences, 2-2-1, Miyama,Funabashi, Chiba 274, Japan Abstract To understand the receptor subtypes responsible for /3adrenoceptormediated relaxa tion of guinea pig taenia caecum, we investigated the effects of isoprenaline and salbutamol . Isoprenaline and salbutamol caused dose-dependent relaxation of the guinea pig taenia caecum. Propranolol, bupranolol and butoxamine produced shifts of the concentration response curves for isoprenaline and salbutamol. Schild regression analyses carried out for propranolol against isoprenaline and salbutamol gave pA2 values of 8.43 and 8.88, respective ly. Schild regression analyses carried out for butoxamine against isoprenaline and sal butamol gave pA2 values of 6.46 and 6.68, respectively. Schild regression analyses carried out for bupranolol against isoprenaline and salbutamol gave pA2 values of 8.60 and 8.69, respectively. However, in the presence of 3 x 10' M atenolol, 10-4 M butoxamine and 10-6 M phentolamine to block the fir , /32 and a -adrenoceptor effects, respectively, Schild regression analyses carried out for bupranolol against isoprenaline and salbutamol gave pA2 values of 5.77 and 5.97, respectively. These results suggest that the relaxant responses to isoprenaline and salbutamol in the guinea pig taenia caecum are mediated by both the /.32 and the A-adrenoceptors. Key words : f2-adrenoceptor, A-adrenoceptor, isoprenaline, salbutamol , guinea pig taenia caecum Introduction The /3adrenoceptors were subclassified as and /32subtypes based on the agonist potency and tissue localization. -

WO 2015/072852 Al 21 May 2015 (21.05.2015) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2015/072852 Al 21 May 2015 (21.05.2015) P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A61K 36/84 (2006.01) A61K 31/5513 (2006.01) kind of national protection available): AE, AG, AL, AM, A61K 31/045 (2006.01) A61P 31/22 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, A61K 31/522 (2006.01) A61K 45/06 (2006.01) BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (21) International Application Number: HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, PCT/NL20 14/050780 KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, (22) International Filing Date: MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, 13 November 2014 (13.1 1.2014) PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, (25) Filing Language: English TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (26) Publication Language: English (84) Designated States (unless otherwise indicated, for every (30) Priority Data: kind of regional protection available): ARIPO (BW, GH, 61/903,430 13 November 2013 (13. 11.2013) US GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, (71) Applicant: RJG DEVELOPMENTS B.V. -
Guidance for the Format and Content of the Protocol of Non-Interventional
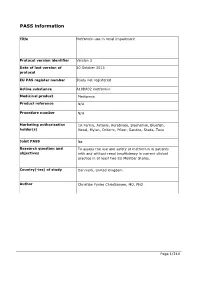
PASS information Title Metformin use in renal impairment Protocol version identifier Version 2 Date of last version of 30 October 2013 protocol EU PAS register number Study not registered Active substance A10BA02 metformin Medicinal product Metformin Product reference N/A Procedure number N/A Marketing authorisation 1A Farma, Actavis, Aurobindo, Biochemie, Bluefish, holder(s) Hexal, Mylan, Orifarm, Pfizer, Sandoz, Stada, Teva Joint PASS No Research question and To assess the use and safety of metformin in patients objectives with and without renal insufficiency in current clinical practice in at least two EU Member States. Country(-ies) of study Denmark, United Kingdom Author Christian Fynbo Christiansen, MD, PhD Page 1/214 Marketing authorisation holder(s) Marketing authorisation N/A holder(s) MAH contact person N/A Page 2/214 1. Table of Contents PASS information .......................................................................................................... 1 Marketing authorisation holder(s) .................................................................................... 2 1. Table of Contents ...................................................................................................... 3 2. List of abbreviations ................................................................................................... 4 3. Responsible parties .................................................................................................... 5 4. Abstract .................................................................................................................. -

Prohibited Substances List
Prohibited Substances List This is the Equine Prohibited Substances List that was voted in at the FEI General Assembly in November 2009 alongside the new Equine Anti-Doping and Controlled Medication Regulations(EADCMR). Neither the List nor the EADCM Regulations are in current usage. Both come into effect on 1 January 2010. The current list of FEI prohibited substances remains in effect until 31 December 2009 and can be found at Annex II Vet Regs (11th edition) Changes in this List : Shaded row means that either removed or allowed at certain limits only SUBSTANCE ACTIVITY Banned Substances 1 Acebutolol Beta blocker 2 Acefylline Bronchodilator 3 Acemetacin NSAID 4 Acenocoumarol Anticoagulant 5 Acetanilid Analgesic/anti-pyretic 6 Acetohexamide Pancreatic stimulant 7 Acetominophen (Paracetamol) Analgesic/anti-pyretic 8 Acetophenazine Antipsychotic 9 Acetylmorphine Narcotic 10 Adinazolam Anxiolytic 11 Adiphenine Anti-spasmodic 12 Adrafinil Stimulant 13 Adrenaline Stimulant 14 Adrenochrome Haemostatic 15 Alclofenac NSAID 16 Alcuronium Muscle relaxant 17 Aldosterone Hormone 18 Alfentanil Narcotic 19 Allopurinol Xanthine oxidase inhibitor (anti-hyperuricaemia) 20 Almotriptan 5 HT agonist (anti-migraine) 21 Alphadolone acetate Neurosteriod 22 Alphaprodine Opiod analgesic 23 Alpidem Anxiolytic 24 Alprazolam Anxiolytic 25 Alprenolol Beta blocker 26 Althesin IV anaesthetic 27 Althiazide Diuretic 28 Altrenogest (in males and gelidngs) Oestrus suppression 29 Alverine Antispasmodic 30 Amantadine Dopaminergic 31 Ambenonium Cholinesterase inhibition 32 Ambucetamide Antispasmodic 33 Amethocaine Local anaesthetic 34 Amfepramone Stimulant 35 Amfetaminil Stimulant 36 Amidephrine Vasoconstrictor 37 Amiloride Diuretic 1 Prohibited Substances List This is the Equine Prohibited Substances List that was voted in at the FEI General Assembly in November 2009 alongside the new Equine Anti-Doping and Controlled Medication Regulations(EADCMR). -

Monoamine Reuptake Inhibitors in Parkinson's Disease
Hindawi Publishing Corporation Parkinson’s Disease Volume 2015, Article ID 609428, 71 pages http://dx.doi.org/10.1155/2015/609428 Review Article Monoamine Reuptake Inhibitors in Parkinson’s Disease Philippe Huot,1,2,3 Susan H. Fox,1,2 and Jonathan M. Brotchie1 1 Toronto Western Research Institute, Toronto Western Hospital, University Health Network, 399 Bathurst Street, Toronto, ON, Canada M5T 2S8 2Division of Neurology, Movement Disorder Clinic, Toronto Western Hospital, University Health Network, University of Toronto, 399BathurstStreet,Toronto,ON,CanadaM5T2S8 3Department of Pharmacology and Division of Neurology, Faculty of Medicine, UniversitedeMontr´ eal´ and Centre Hospitalier de l’UniversitedeMontr´ eal,´ Montreal,´ QC, Canada Correspondence should be addressed to Jonathan M. Brotchie; [email protected] Received 19 September 2014; Accepted 26 December 2014 Academic Editor: Maral M. Mouradian Copyright © 2015 Philippe Huot et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. The motor manifestations of Parkinson’s disease (PD) are secondary to a dopamine deficiency in the striatum. However, the degenerative process in PD is not limited to the dopaminergic system and also affects serotonergic and noradrenergic neurons. Because they can increase monoamine levels throughout the brain, monoamine reuptake inhibitors (MAUIs) represent potential therapeutic agents in PD. However, they are seldom used in clinical practice other than as antidepressants and wake-promoting agents. This review article summarises all of the available literature on use of 50 MAUIs in PD. The compounds are divided according to their relative potency for each of the monoamine transporters. -

Novel Method of Administering Β-Blockers and Novel Dosage Forms Containing Same Anwar A
University of Kentucky UKnowledge Pharmaceutical Sciences Faculty Patents Pharmaceutical Sciences 1-31-1984 Novel Method of Administering β-Blockers and Novel Dosage Forms Containing Same Anwar A. Hussain University of Kentucky Right click to open a feedback form in a new tab to let us know how this document benefits oy u. Follow this and additional works at: https://uknowledge.uky.edu/ps_patents Part of the Pharmacy and Pharmaceutical Sciences Commons Recommended Citation Hussain, Anwar A., "Novel Method of Administering β-Blockers and Novel Dosage Forms Containing Same" (1984). Pharmaceutical Sciences Faculty Patents. 124. https://uknowledge.uky.edu/ps_patents/124 This Patent is brought to you for free and open access by the Pharmaceutical Sciences at UKnowledge. It has been accepted for inclusion in Pharmaceutical Sciences Faculty Patents by an authorized administrator of UKnowledge. For more information, please contact [email protected]. Unlted States Patent [191 [11] 4,428,883 Hussain [45] Jan. 31, 1984 [54] NOVEL METHOD OF ADMINISTERING 4,012,444 3/l977 Lunts ................................. .. 424/ 324 B-BLOCKERSF0 RMS Co NTA' AND, INING NOVEL SAME DOSAGE ' 4,250,163, , 1.1’;2/l981 llieoll’loldNagaioe a ......................................... ---- -~.. .. 424/14 [7 5 ] I nventor : An war A . Hussam,° Lexington' , Ky . FOREIGN PATENT DOCUMENTS [73] Assignee: The University of Kentucky Research . Foundation’ Lexington, Ky. 979389 1/ 1965 United Klngdom . I [21] APPL Nod 241,413 OTHER PUBLICATIONS . Stern, Arzmit. Forsch, vol. 24, 1974, pp. 70-71. [22] Flled‘ Mm‘- 6’ 1981 Black, Brit. J. PharmacoL, (1965) 25, pp. 577-591. [51] Int. Cl.3 ................... .. A61K 27/00; A61K 31/15; Prima'y Examiner_stanley L Friedman A61K 31/40; A61K 31/47; A61K 31/135; Attorney, Agent, or Firm-Burns, Doane, Swecker & A61K 31/165; A61K 31/435; A61K 31/475; Mathis A61K 47/00; C07C 143/90; CllD 1/28; C09F - 5 /()() [57] ABSTRACT [52] US. -

Official Protocol Title: NCT Number: NCT02750761
Official Protocol Title: A Phase 1, Single-Administration Pharmacokinetic and Safety Study of Oral and IV Tedizolid Phosphate in Hospitalized Subjects 2 to <12 Years Old NCT number: NCT02750761 Document Date: 23-Jun-2017 Tedizolid Phosphate (MK-1986) 1 Protocol TR701-120/MK-1986-013, Amendment 4 THIS PROTOCOL AND ALL OF THE INFORMATION RELATING TO IT ARE CONFIDENTIAL AND PROPRIETARY PROPERTY OF MERCK SHARP & DOHME CORP., A SUBSIDIARY OF MERCK & CO., INC., WHITEHOUSE STATION, NJ, U.S.A. SPONSOR: Cubist Pharmaceuticals, LLC, A wholly-owned indirect subsidiary of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc. (hereafter referred to as the Sponsor or Merck) Weystrasse 20, Lucerne 6 Switzerland Protocol-specific Sponsor Contact information can be found in the Investigator Trial File Binder (or equivalent). TITLE: A Phase 1, Single-Administration Pharmacokinetic and Safety Study of Oral and IV Tedizolid Phosphate in Hospitalized Subjects 2 to <12 Years Old IND NUMBER: [106,307 (IV) and 125,076 (Oral Suspension)] EudraCT NUMBER: 2015-004595-29 23-Jun-2017 Confidential 04PPD9 Tedizolid Phosphate (MK-1986) 2 Protocol TR701-120, Amendment 4 SUMMARY OF CHANGES: TR701-120 Amendment 4 PRIMARY REASON(S) FOR THIS AMENDMENT: Section Change Rationale 1.0 Synopsis Updated dose for 6 to <12 years from 5 mg/kg to The dose level for the two age groups was adjusted Methodology; 4mg/kg, dose for 2 to <6 years from 5 mg/kg to 6 based on the results of the first interim analysis of the Investigational mg/kg based on the data from interim safety and safety and pharmacokinetic data. -
![Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set](https://docslib.b-cdn.net/cover/8870/ehealth-dsi-ehdsi-v2-2-2-or-ehealth-dsi-master-value-set-1028870.webp)
Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set
MTC eHealth DSI [eHDSI v2.2.2-OR] eHealth DSI – Master Value Set Catalogue Responsible : eHDSI Solution Provider PublishDate : Wed Nov 08 16:16:10 CET 2017 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 1 of 490 MTC Table of Contents epSOSActiveIngredient 4 epSOSAdministrativeGender 148 epSOSAdverseEventType 149 epSOSAllergenNoDrugs 150 epSOSBloodGroup 155 epSOSBloodPressure 156 epSOSCodeNoMedication 157 epSOSCodeProb 158 epSOSConfidentiality 159 epSOSCountry 160 epSOSDisplayLabel 167 epSOSDocumentCode 170 epSOSDoseForm 171 epSOSHealthcareProfessionalRoles 184 epSOSIllnessesandDisorders 186 epSOSLanguage 448 epSOSMedicalDevices 458 epSOSNullFavor 461 epSOSPackage 462 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 2 of 490 MTC epSOSPersonalRelationship 464 epSOSPregnancyInformation 466 epSOSProcedures 467 epSOSReactionAllergy 470 epSOSResolutionOutcome 472 epSOSRoleClass 473 epSOSRouteofAdministration 474 epSOSSections 477 epSOSSeverity 478 epSOSSocialHistory 479 epSOSStatusCode 480 epSOSSubstitutionCode 481 epSOSTelecomAddress 482 epSOSTimingEvent 483 epSOSUnits 484 epSOSUnknownInformation 487 epSOSVaccine 488 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 3 of 490 MTC epSOSActiveIngredient epSOSActiveIngredient Value Set ID 1.3.6.1.4.1.12559.11.10.1.3.1.42.24 TRANSLATIONS Code System ID Code System Version Concept Code Description (FSN) 2.16.840.1.113883.6.73 2017-01 A ALIMENTARY TRACT AND METABOLISM 2.16.840.1.113883.6.73 2017-01