Investigation of Isotopic Signatures for Sources of Groundwater Contamination at the Hanford Site
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Per-10 Basic Information Relating to Uranium
PER-10 1 BASIC INFORMATION RELATING TO URANIUM-ENRICHMENT CALCULATIONS AND FUEL REQUIREMENTS FOR NUCLEAR POWER REACTORS by K.T. Brown m 3 ATOMIC ENERGY BOARD Pelindaba PRETORIA Republic of South Africa February 1977 :::: : =:::""""""::::::;::: i:::""""""" :::::::::i:::::::::::::::H:::""»"""::::::::::::::::: BASIC INFORMATION RELATING TO URANIUM-ENRICHMENT CALCULATIONS AND FUEL REQUIREMENTS FOR NUCLEAR POWER REACTORS hy K.T. Brown POSTAL ADDRESS: Atomic Energy Board Private Bag X256 PRETORIA 0001 PELINDABA Fi-ln liai v 1977 ISBN U 86Ü6U 654 9 Pago Page SAMEVATTING 2 ABSTRACT 2 3. REACTOR FUEL REQUIREMENTS 5 1. INTRODUCTION 3 3.1 Reactor Types 5 2. URANIUM ENRICHMENT 3 3.1.1 Pressurised-water roactor 5 2.1 Definitions 3 3.1.2 Boiling-water reactor 5 2.1.1 Natural uranium 3 3.1.3 CANDU-PHW 6 2.1.2 Fissile 3 3.1.4 High-temperature gas-cooled reactor 6 2.1.3 Fertile 3 2.1.5 Liquid-metal-cooled fast breeder reactor ... .6 2.1.4 Enrichment 3 3.2 Nuclear Fuel Cycles 6 2.1.5 Product 3 3.3 Typical Fuel Requirements 6 2.1.6 Feed 3 3.3.1 Pressurised-wator reactor 7 2.1.7 Tails, or waste 3 3.3.2 Boiling-water reactor 8 2.1.8 Cascade 3 3.3.3 CANDU-PHW 9 2.1.9 Separative work 4 3.3.4 High-temperature gas-cooled reactor 9 2.1.10 Separative-work unit 4 3.3.5 Liquid-metal-cooled fast breeder reactor ... 10 2.2 Enrichment Parameters 4 3.3.6 Comparative data 10 2.3 Optimum Tails Assay 5 4. -

High Accuracy Measurement of Isotope Ratios of Molybdenum in Some Terrestrial Molybdenites
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector ARTICLES High Accuracy Measurement of Isotope Ratios of Molybdenum in Some Terrestrial Molybdenites Qi-Lu* and Akimasa Masuda Department of Chemistry, Faculty of Science, The University of Tokyo, Tokyo, Japan The isotope ratios of molybdenum in molybdenites were studied. A special triple filament technique was used to obtain stable and lasting signals for MO+. There are no differences bigger than ~0.4 parts per IO4 among four samples and the standard. CJ Am Sot Mass Spectrom 1992, 3, IO- 17) olybdenum is a very interesting element be- denum thus far, in spite of the potential importance cause its seven isotopes can reflect several of research in isotopic abundance of molybdenum. M effects related to nuclear physics. The nu- In this study we have established a method for clear phenomena that may affect the isotope ratios in securing stable and lasting current of MO+ and exam- question are (1) the synthesis of seven isotopes of MO ined the mass fractionation of MO isotopes during involving three processes (r, s, and p) in the standard measurement. Based on these studies, the isotope model of nucleosynthesis [I]; ($2 the nuclear hssion of ratios of MO were determined with high precision uranium-238, which produces MO, “MO, 98Mo, and for some molybdenites from a variety of locations ‘“MO; and (3) the double-beta decays of ‘“Zr and throughout the world. The present study will afford a lwMo leading to 96Mo and ‘“Ru. Another intriguing foundation for further precise studies of molybdenum property of this element is that the anomalous abun- isotopes involving meteorites and terrestrial rocks. -

Progress and Challenges in Using Stable Isotopes to Trace Plant Carbon and Water Relations Across Scales
Biogeosciences, 9, 3083–3111, 2012 www.biogeosciences.net/9/3083/2012/ Biogeosciences doi:10.5194/bg-9-3083-2012 © Author(s) 2012. CC Attribution 3.0 License. Progress and challenges in using stable isotopes to trace plant carbon and water relations across scales C. Werner1,19, H. Schnyder2, M. Cuntz3, C. Keitel4, M. J. Zeeman5, T. E. Dawson6, F.-W. Badeck7, E. Brugnoli8, J. Ghashghaie9, T. E. E. Grams10, Z. E. Kayler11, M. Lakatos12, X. Lee13, C. Maguas´ 14, J. Ogee´ 15, K. G. Rascher1, R. T. W. Siegwolf16, S. Unger1, J. Welker17, L. Wingate18, and A. Gessler11 1Experimental and Systems Ecology, University Bielefeld, 33615 Bielefeld, Germany 2Lehrstuhl fur¨ Grunlandlehre,¨ Technische Universitat¨ Munchen,¨ 85350 Freising-Weihenstephan, Germany 3UFZ – Helmholtz Centre for Environmental Research, Permoserstr. 15, 04318 Leipzig, Germany 4University of Sydney, Faculty of Agriculture, Food and Natural Resources, 1 Central Avenue, Eveleigh, NSW 2015, Australia 5College of Oceanic and Atmospheric Sciences, Oregon State University, 104 COAS Admin Bldg, Corvallis (OR), USA 6Center for Isotope Biogeochemistry, Department of Integrative Biology, University of California, Berkeley, CA 94720, USA 7Potsdam Institute for Climate Impact Research (PIK) PF 60 12 03, 14412 Potsdam, Germany 8CNR – National Research Council of Italy – Institute of Agro-environmental and Forest Biology, via Marconi 2, 05010 Porano (TR), Italy 9Laboratoire d’Ecologie, Systematique´ et Evolution (ESE), CNRS AgroParisTech – UMR8079, Batimentˆ 362, Universite´ de Paris-Sud (XI), 91405 Orsay Cedex, France 10Ecophysiology of Plants, Department of Ecology and Ecosystem Management, Technische Universitat¨ Munchen,¨ Von-Carlowitz-Platz 2, 85354 Freising, Germany 11Institute for Landscape Biogeochemistry Leibniz-Zentrum fur¨ Agrarlandschaftsforschung (ZALF) e.V., Eberswalderstr. -

Isotope Production Potential at Sandia National Laboratories: Product, Waste, Packaging, and Transportation*
Isotope Production Potential at Sandia National Laboratories: Product, Waste, Packaging, and Transportation* A. J. Trennel Transportation Systems Department *- *-, o / /"-~~> Sandia National Laboratories** ' J Albuquerque, NM 87185 O Q T » Abstract The U.S. Congress directed the U.S. Department of Energy to establish a domestic source of molybdenum-99, an essential isotope used in nuclear medicine and radiopharmacology. An Environmental Impact Statement for production of 99Mo at one of four candidate sites is being prepared. As one of the candidate sites, Sandia National Laboratories is developing the Isotope Production Project. Using federally approved processes and procedures now owned by the U.S. Department of Energy, and existing facilities that would be modified to meet the production requirements, the Sandia National Laboratories' Isotope Project would manufacture up to 30 percent of the U.S. market, with the capacity to meet 100 percent of the domestic need if necessary. This paper provides a brief overview of the facility, equipment, and processes required to produce isotopes. Packaging and transportation issues affecting both product and waste are addressed, and the storage and disposal of the four low-level radioactive waste types generated by the production program are considered. Recommendations for future development are provided. This work was performed at Sandia National Laboratories, Albuquerque, New Mexico, for the U.S. Department of Energy under Contract DE-AC04-94AL85000. A U.S. Department of Energy facility. DISTRPJTO OF THIS DOCUMENT IS UNLIMITED #t/f W A8 1 fcll PROJECT NEED AND BACKGROUND Nuclear medicine is an expanding segment of today's medical and pharmaceutical communities. Specific radioactive isotopes are vital, with molybdenum-99 (99Mo) being the most important medical isotope. -

Oxygen Isotopic Signature of CO2 from Combustion Processes
Atmos. Chem. Phys., 11, 1473–1490, 2011 www.atmos-chem-phys.net/11/1473/2011/ Atmospheric doi:10.5194/acp-11-1473-2011 Chemistry © Author(s) 2011. CC Attribution 3.0 License. and Physics Oxygen isotopic signature of CO2 from combustion processes M. Schumacher1,2,3, R. A. Werner3, H. A. J. Meijer1, H. G. Jansen1, W. A. Brand2, H. Geilmann2, and R. E. M. Neubert1 1Centre for Isotope Research, University of Groningen, Nijenborgh 4, 9747 AG Groningen, The Netherlands 2Max-Planck-Institute for Biogeochemistry, Hans-Knoell-Straße 10, 07745, Jena, Germany 3Institute of Plant Sciences, Swiss Federal Institute of Technology Zurich,¨ Universitatsstraße¨ 2, 8092 Zurich,¨ Switzerland Received: 21 July 2008 – Published in Atmos. Chem. Phys. Discuss.: 5 November 2008 Revised: 2 February 2011 – Accepted: 5 February 2011 – Published: 16 February 2011 Abstract. For a comprehensive understanding of the global (diffusive) transport of oxygen to the reaction zone as the carbon cycle precise knowledge of all processes is neces- cause of the isotope fractionation. The original total 18O sig- sary. Stable isotope (13C and 18O) abundances provide in- nature of the material appeared to have little influence, how- formation for the qualification and the quantification of the ever, a contribution of specific bio-chemical compounds to diverse source and sink processes. This study focuses on the individual combustion products released from the involved 18 δ O signature of CO2 from combustion processes, which material became obvious. are widely present both naturally (wild fires), and human in- duced (fossil fuel combustion, biomass burning) in the car- bon cycle. All these combustion processes use atmospheric 1 Introduction oxygen, of which the isotopic signature is assumed to be con- stant with time throughout the whole atmosphere. -

Uranium Fact Sheet
Fact Sheet Adopted: December 2018 Health Physics Society Specialists in Radiation Safety 1 Uranium What is uranium? Uranium is a naturally occurring metallic element that has been present in the Earth’s crust since formation of the planet. Like many other minerals, uranium was deposited on land by volcanic action, dissolved by rainfall, and in some places, carried into underground formations. In some cases, geochemical conditions resulted in its concentration into “ore bodies.” Uranium is a common element in Earth’s crust (soil, rock) and in seawater and groundwater. Uranium has 92 protons in its nucleus. The isotope2 238U has 146 neutrons, for a total atomic weight of approximately 238, making it the highest atomic weight of any naturally occurring element. It is not the most dense of elements, but its density is almost twice that of lead. Uranium is radioactive and in nature has three primary isotopes with different numbers of neutrons. Natural uranium, 238U, constitutes over 99% of the total mass or weight, with 0.72% 235U, and a very small amount of 234U. An unstable nucleus that emits some form of radiation is defined as radioactive. The emitted radiation is called radioactivity, which in this case is ionizing radiation—meaning it can interact with other atoms to create charged atoms known as ions. Uranium emits alpha particles, which are ejected from the nucleus of the unstable uranium atom. When an atom emits radiation such as alpha or beta particles or photons such as x rays or gamma rays, the material is said to be undergoing radioactive decay (also called radioactive transformation). -

Investigations of Nuclear Forensic Signatures in Uranium Bearing Materials a Dissertation Submitted to the Graduate School of the University of Cincinnati
Investigations of Nuclear Forensic Signatures in Uranium Bearing Materials A dissertation submitted to the Graduate School of the University of Cincinnati in partial fulfillment of the requirements for the degree of Doctor of Philosophy (Ph.D) In the Department of Chemistry Of the McMicken College of Arts and Sciences By Lisa Ann Meyers B.S. Ohio Northern University 2009 August 2013 Committee Chairs: Thomas Beck, Ph.D. Apryll Stalcup, Ph.D. i Abstract Nuclear forensics is a multidisciplinary science that uses a variety of analytical methods and tools to investigate the physical, chemical, elemental, and isotopic characteristics of nuclear and radiological material. A collection of these characteristics is called signatures that aids in determining how, where and when the material was manufactured. Radiological chronometry (i.e., age dating) is an important tool in nuclear forensics that uses several methods to determine the length of time that has elapsed since a material was last purified. For example, the “age” of a uranium-bearing material is determined by measuring the ingrowth of 230Th from its parent, 234U. A piece of scrap uranium metal bar buried in the dirt floor of an old, abandoned metal rolling mill was analyzed using multi-collector inductively coupled plasma mass spectroscopy (MC-ICP- MS). The mill rolled uranium rods in the 1940s and 1950s. The age of the metal bar was determined to be 61 years at the time of analysis using the 230Th/234U chronometer, which corresponds to a purification date of July 1950 ± 1.5 years. Radiochronometry was determined for three different types of uranium metal samples. -

An Advanced Sodium-Cooled Fast Reactor Core Concept Using Uranium-Free Metallic Fuels for Maximizing TRU Burning Rate
sustainability Article An Advanced Sodium-Cooled Fast Reactor Core Concept Using Uranium-Free Metallic Fuels for Maximizing TRU Burning Rate Wuseong You and Ser Gi Hong * Department of Nuclear Engineering, Kyung Hee University, Deogyeong-daero, GiHeung-gu, Yongin, Gyeonggi-do 446-701, Korea; [email protected] * Correspondence: [email protected]; Tel.: +82-31-201-2782 Received: 24 October 2017; Accepted: 28 November 2017; Published: 1 December 2017 Abstract: In this paper, we designed and analyzed advanced sodium-cooled fast reactor cores using uranium-free metallic fuels for maximizing burning rate of transuranics (TRU) nuclides from PWR spent fuels. It is well known that the removal of fertile nuclides such as 238U from fuels in liquid metal cooled fast reactor leads to the degradation of important safety parameters such as the Doppler coefficient, coolant void worth, and delayed neutron fraction. To resolve the degradation of the Doppler coefficient, we considered adding resonant nuclides to the uranium-free metallic fuels. The analysis results showed that the cores using uranium-free fuels loaded with tungsten instead of uranium have a significantly lower burnup reactivity swing and more negative Doppler coefficients than the core using uranium-free fuels without resonant nuclides. In addition, we considered the use of axially central B4C absorber region and moderator rods to further improve safety parameters such as sodium void worth, burnup reactivity swing, and the Doppler coefficient. The results of the analysis showed that the final design core can consume ~353 kg per cycle and satisfies self-controllability under unprotected accidents. The fuel cycle analysis showed that the PWR–SFR coupling fuel cycle option drastically reduces the amount of waste going to repository and the SFR burner can consume the amount of TRUs discharged from 3.72 PWRs generating the same electricity. -

12 Natural Isotopes of Elements Other Than H, C, O
12 NATURAL ISOTOPES OF ELEMENTS OTHER THAN H, C, O In this chapter we are dealing with the less common applications of natural isotopes. Our discussions will be restricted to their origin and isotopic abundances and the main characteristics. Only brief indications are given about possible applications. More details are presented in the other volumes of this series. A few isotopes are mentioned only briefly, as they are of little relevance to water studies. Based on their half-life, the isotopes concerned can be subdivided: 1) stable isotopes of some elements (He, Li, B, N, S, Cl), of which the abundance variations point to certain geochemical and hydrogeological processes, and which can be applied as tracers in the hydrological systems, 2) radioactive isotopes with half-lives exceeding the age of the universe (232Th, 235U, 238U), 3) radioactive isotopes with shorter half-lives, mainly daughter nuclides of the previous catagory of isotopes, 4) radioactive isotopes with shorter half-lives that are of cosmogenic origin, i.e. that are being produced in the atmosphere by interactions of cosmic radiation particles with atmospheric molecules (7Be, 10Be, 26Al, 32Si, 36Cl, 36Ar, 39Ar, 81Kr, 85Kr, 129I) (Lal and Peters, 1967). The isotopes can also be distinguished by their chemical characteristics: 1) the isotopes of noble gases (He, Ar, Kr) play an important role, because of their solubility in water and because of their chemically inert and thus conservative character. Table 12.1 gives the solubility values in water (data from Benson and Krause, 1976); the table also contains the atmospheric concentrations (Andrews, 1992: error in his Eq.4, where Ti/(T1) should read (Ti/T)1); 2) another category consists of the isotopes of elements that are only slightly soluble and have very low concentrations in water under moderate conditions (Be, Al). -
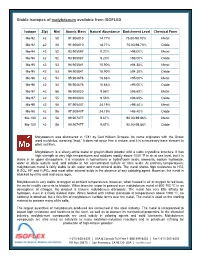
Stable Isotopes of Molybdenum Available from ISOFLEX
Stable isotopes of molybdenum available from ISOFLEX Isotope Z(p) N(n) Atomic Mass Natural Abundance Enrichment Level Chemical Form Mo-92 42 50 91.906810 14.77% 75.00-98.70% Metal Mo-92 42 50 91.906810 14.77% 75.00-98.70% Oxide Mo-94 42 52 93.905087 9.23% >98.00% Metal Mo-94 42 52 93.905087 9.23% >98.00% Oxide Mo-95 42 53 94.905841 15.90% ≥94.30% Metal Mo-95 42 53 94.905841 15.90% ≥94.30% Oxide Mo-96 42 54 95.904678 16.68% >95.00% Metal Mo-96 42 54 95.904678 16.68% >95.00% Oxide Mo-97 42 55 96.906020 9.56% ≥96.60% Metal Mo-97 42 55 96.906020 9.56% ≥96.60% Oxide Mo-98 42 56 97.905407 24.19% >98.40% Metal Mo-98 42 56 97.905407 24.19% >98.40% Oxide Mo-100 42 58 99.907477 9.67% 90.00-99.86% Metal Mo-100 42 58 99.907477 9.67% 90.00-99.86% Oxide Molybdenum was discovered in 1781 by Carl William Scheele. Its name originates with the Greek word molybdos, meaning “lead.” It does not occur free in nature, and it is a necessary trace element in plant nutrition. Molybdenum is a silvery-white metal or grayish-black powder with a cubic crystalline structure. It has high strength at very high temperatures and oxidizes rapidly above 1000 °F in air at sea level, but it is stable in an upper atmosphere. -

The Nuclear Fuel Cycle
THE COLLECTION > From the uranium mine> toI wNTasRtOeD dUisCpToIsOaN l 1 > The atom 2 > Radioactivity 3 > Radiation and man 4 > Energy 5 > Nuclear energy: fusion and fission 6 > How a nuclear reactor works 7 > The nuclear fuel cycle 7 > The nuclear fuel cycle FROM RESEARCH 8 > Microelectronics 9 > The laser: a concentrate of light TO INDUSTRY 10 > Medical imaging 11 > Nuclear astrophysics 12 > Hydrogen 7 >>TThhee nnuucclleeaarr ffuueell ccyyccllee UPSTREAM THE REACTOR: PREPARING THE FUEL IN THE REACTOR: FUEL CONSUMPTION DOWNSTREAM THE REACTOR: REPROCESSING NUCLEAR WASTE NUCLEAR WASTE © Commissariat à l’’Énergie Atomique et aux Energies Alternatives, 2005 Communication Division Bâtiment Siège - 91191 Gif-sur-Yvette cedex www.cea.fr ISSN 1637-5408. From the uranium mine to waste disposal 7 > The nuclear fuel cycle From the uranium mine to waste disposal 7 > The nuclear fuel cycle 2 > CONTENTS > INTRODUCTION 3 Uranium ore is extracted from open-pit mines – such as the McClear mines in Canada seen here – or underground workings. a m e g o C © “The nuclear fuel cycle includes an erray UPSTREAM THE REACTOR: of industrial operations, from uranium PREPARING THE FUEL 4 e mining to the disposal of radioactive l Extracting uranium from the ore 5 waste.” c Concentrating and refining uranium 6 y Enriching uranium 6 c Enrichment methods 8 l introduction uel is a material that can be burnt to pro - IN THE REACTOR: FUEL CONSUMPTION 9 Fvide heat. The most familiar fuels are wood, e Preparing fuel assemblies 10 coal, natural gas and oil. By analogy, the ura - e g a nium used in nuclear power plants is called Per unit or mass (e.g. -

PSI • Scientific Report 1999 /Volume I
CH0000002 PAUL SCHERRER INSTITUT ISSN 1423-7296 March 2000 PSI • Scientific Report 1999 /Volume I Particles and Matter 3 1/28 An international collaboration of radiochemists led by the Laboratory for Radiochemistry and Environmental Chemistry of PSl and Bern University succeeded for the first time to experimentally investigate the chemical properties of bohrium (element 107) and to establish it as a member of group 7 of the Periodic Table. During a one-month long experiment, a total of only six bohrium atoms were gaschromatographically isolated in the form of volatile bohrium oxychloride molecules and identified by registering their unique decay sequence of alpha particle emissions via dubnium (element 105) to lawrencium (element 103). The short-lived bohrium atoms, decaying with a half-life of about 20 s, were produced by bombarding a very rare, highly radioactive berkelium target supplied by the US department of energy with an intense beam of neon ions at the PSl injector 1 cyclotron. PAUL SCHERRER INSTITUT ISSN 1423-7296 March 2000 \} Scientific Report 1999 Volume I Particles and Matter ed. by: J. Gobrecht, H. Gaggeler, D. Herlach, K. Junker, P.-R. Kettle, P. Kubik, A. Zehnder CH-5232 Villigen PSI Switzerland Telephone:+41 56 310 21 11 Telefax:+ 41 56 310 21 99 http://www.psi.ch TABLE OF CONTENTS Introduction .1 Laboratory for Particle Physics 3 Foreword 4 Particle Physics Theory Theory (I) 5 Theory (II) 6 Ring Accelerator Experiments Particle Properties and Decays A precise measurement of the Jt+-^7t°e+v decay rate 7 Measurement of