Movement and Habitat Use of Hatchery-Reared Juvenile Robust Redhorse Moxostoma Robustum Released
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lloyd Shoals
Southern Company Generation. 241 Ralph McGill Boulevard, NE BIN 10193 Atlanta, GA 30308-3374 404 506 7219 tel July 3, 2018 FERC Project No. 2336 Lloyd Shoals Project Notice of Intent to Relicense Lloyd Shoals Dam, Preliminary Application Document, Request for Designation under Section 7 of the Endangered Species Act and Request for Authorization to Initiate Consultation under Section 106 of the National Historic Preservation Act Ms. Kimberly D. Bose, Secretary Federal Energy Regulatory Commission 888 First Street, N.E. Washington, D.C. 20426 Dear Ms. Bose: On behalf of Georgia Power Company, Southern Company is filing this letter to indicate our intent to relicense the Lloyd Shoals Hydroelectric Project, FERC Project No. 2336 (Lloyd Shoals Project). We will file a complete application for a new license for Lloyd Shoals Project utilizing the Integrated Licensing Process (ILP) in accordance with the Federal Energy Regulatory Commission’s (Commission) regulations found at 18 CFR Part 5. The proposed Process, Plan and Schedule for the ILP proceeding is provided in Table 1 of the Preliminary Application Document included with this filing. We are also requesting through this filing designation as the Commission’s non-federal representative for consultation under Section 7 of the Endangered Species Act and authorization to initiate consultation under Section 106 of the National Historic Preservation Act. There are four components to this filing: 1) Cover Letter (Public) 2) Notification of Intent (Public) 3) Preliminary Application Document (Public) 4) Preliminary Application Document – Appendix C (CEII) If you require further information, please contact me at 404.506.7219. Sincerely, Courtenay R. -

Of Georgia Water Coalition Re Draft Environmental Impact Statement
GIEORGIA WATER COALITION '-Fl FC- C-3, November 28, 2007 Ti Chief, Rules and Directives Branch I-F-i --A Division of Administrative Services '7/P//tP 7 IC Office of Administration Mailstop T-6D59 1~2fX~ ~ U.S. Nuclear Regulatory Commission Washington, DC 20555-0001 E-mail: [email protected] 0 RE: Draft Environmental Impact Statement for the Plant Vogtle Early Site Permit To Whom It May Concern: The Georgia Water Coalition ("GWC" or "the Coalition") wishes to submit the following comments concerning the draft Environmental Impact Statement ("DEIS") for Plant Vogtle's Early Site Permit ("ESP"). The GWC is composed of 150 conservation, recreation, evangelical, civic and business organizations and represents hundreds of thousands of Georgians; a list of member organizations is attached to this letter. The Coalition is dedicated to the protection of water quality and water resources throughout the state. We have serious concerns about the implications of the expansion of Plant Vogtle on the water quality and stream flow of the Savannah River and its tributaries and believe that the DEIS is deficient in adequately addressing those concerns. Additionally, four of our member groups including Atlanta WAND, Center for a Sustainable Coast, Savannah Riverkeeper, and Southern Alliance for Clean Energy, have challenged the legality of the ESP, which raises several important water related issues. In our previously filed scoping comments, we asked that the Nuclear Regulatory Commission ("NRC") conduct a thorough environmental review of the direct, indirect and cumulative impacts of the proposed expansion on the Savannah River's ecology and on the local economies of downstream communities. -

Stream-Temperature Charcteristics in Georgia
STREAM-TEMPERATURE CHARACTERISTICS IN GEORGIA U.S. GEOLOGICAL SURVEY Prepared in cooperation with the GEORGIA DEPARTMENT OF NATURAL RESOURCES ENVIRONMENTAL PROTECTION DIVISION Water-Resources Investigations Report 96-4203 STREAM-TEMPERATURE CHARACTERISTICS IN GEORGIA By T.R. Dyar and S.J. Alhadeff ______________________________________________________________________________ U.S. GEOLOGICAL SURVEY Water-Resources Investigations Report 96-4203 Prepared in cooperation with GEORGIA DEPARTMENT OF NATURAL RESOURCES ENVIRONMENTAL PROTECTION DIVISION Atlanta, Georgia 1997 U.S. DEPARTMENT OF THE INTERIOR BRUCE BABBITT, Secretary U.S. GEOLOGICAL SURVEY Charles G. Groat, Director For additional information write to: Copies of this report can be purchased from: District Chief U.S. Geological Survey U.S. Geological Survey Branch of Information Services 3039 Amwiler Road, Suite 130 Denver Federal Center Peachtree Business Center Box 25286 Atlanta, GA 30360-2824 Denver, CO 80225-0286 CONTENTS Page Abstract . 1 Introduction . 1 Purpose and scope . 2 Previous investigations. 2 Station-identification system . 3 Stream-temperature data . 3 Long-term stream-temperature characteristics. 6 Natural stream-temperature characteristics . 7 Regression analysis . 7 Harmonic mean coefficient . 7 Amplitude coefficient. 10 Phase coefficient . 13 Statewide harmonic equation . 13 Examples of estimating natural stream-temperature characteristics . 15 Panther Creek . 15 West Armuchee Creek . 15 Alcovy River . 18 Altamaha River . 18 Summary of stream-temperature characteristics by river basin . 19 Savannah River basin . 19 Ogeechee River basin. 25 Altamaha River basin. 25 Satilla-St Marys River basins. 26 Suwannee-Ochlockonee River basins . 27 Chattahoochee River basin. 27 Flint River basin. 28 Coosa River basin. 29 Tennessee River basin . 31 Selected references. 31 Tabular data . 33 Graphs showing harmonic stream-temperature curves of observed data and statewide harmonic equation for selected stations, figures 14-211 . -

Georgia Power Study Plan Meeting Presentations
Lloyds Shoals Study Plan Meeting (FERC No. 2336) January 16, 2019 Introduction Courtenay O’Mara, P.E. Southern Company 2 Study Plan Meeting Agenda Morning Session: 10:00 a.m. ‒ 12:00 p.m. • Welcome, Introductions & Operations Presentation 10:00 – 10:30 a.m. • Recreation and Land Use Study 10:30 – 11:00 a.m. • Terrestrial, Wetland, and Riparian Resources Study 11:00 – 11:30 a.m. • Rare, Threatened, and Endangered Species Study 11:30 a.m. – 12:00 p.m. Lunch: 12:00 p.m. ‒ 1:00 p.m. Afternoon Session: 1:00 p.m. ‒ 5:00 p.m. • Water Resources Study 1:00 – 1:30 p.m. • Fish and Aquatic Resources Study 1:30 – 2:00 p.m. • American Eel Abundance & Upstream Movements Study 2:00 – 2:30 p.m. • Geology and Soils Study 2:30 – 3:00 p.m. • Cultural Resources Study 3:00 – 3:30 p.m. • Q&A Discussion (if none, Early Dismissal at 3:30 p.m.) 3:30 – 5:00 p.m. 3 Study Plan Development Schedule Mar 20, 2019 Nov 5, 2018 Dec 20, 2018 Jan 16, 2019 45 Comments on GPC Files 90 Comments PAD and SD1, Proposed Study on 30 and Study Plan 30 Study Plan Proposed Requests FERC Issues SD2 Meeting Study Plan if Necessary DUE DATE Resolution of Study Issues Apr 19, 2019 May 20, 2019 May 2019-Apr 2020 GPC Files 30 20 FERC Issues No Disputes Revised Study Plan First Study Plan Determination Jun 10, 2019 Season File Reply 20 70 Studies Notice of Study Dispute Comments within Study Dispute Resolution 15 days Process If Necessary If Necessary 4 Content of Study Request (18 CFR § 5.9(b)) 1. -
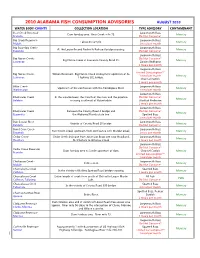
2010 Alabama Fish Consumption Advisories
2010 ALABAMA FISH CONSUMPTION ADVISORIES AUGUST 2010 WATER BODY-COUNTY COLLECTION LOCATION TYPE ADVISORY CONTAMINANT Bear Creek Reservoir - Largemouth Bass Dam forebay area. Bear Creek mile 75. Mercury Franklin Do Not Consume* Big Creek Reservoir - Largemouth Bass Lakewide sample. Mercury Mobile 1 meal per month Big Escambia Creek - Largemouth Bass At the Louisville and Nashville Railroad bridge crossing. Mercury Escambia Do Not Consume* Largemouth Bass Big Nance Creek- Do Not Consume* Big Nance Creek at Lawrence County Road 25. Mercury Lawrence Golden Redhorse 2 meals per month Largemouth Bass Limited Consumption** Big Nance Creek- Wilson Reservoir. Big Nance Creek embayment upstream of AL 1 meal per month Mercury Lawrence Highway 101 bridge. Channel Catfish 2 meals per month Bilbo Creek - Largemouth B ass Upstream of the confluence with the Tombigbee River. Mercury Washington 1 meal per month Largemouth Bass Blackwater Creek- In the area between the mouth of the river and the pipeline Do Not Consume* Mercury Baldwin crossing southeast of Robertsdale. Blacktail Redhorse 2 meals per month Largemouth Bass Blackwater Creek- Between the County Road 4 bridge and Do Not Consume* Mercury Escambia the Alabama/Florida state line. Spotted Bass 1 m eal per month Bon Secour River - Largemouth Bass Vicinity of County Road 10 bridge. Mercury Baldwin Do Not Consume* Burnt Corn Creek - Largemouth Bass Burnt Corn Creek upstream from confluence with Murder Creek. Mercury Escambia 1 meal per month Cedar Creek - Cedar Creek drainage from American Brass site near Headland, Largemouth Bass Mercury Houston AL tributary to Omusee Creek . 2 meals per month Largemouth Bass Do Not Consume* Cedar Creek Reservoir- Dam forebay area to 1 mile upstream of dam. -

Normal Streamflows and Water Levels: Summary of Hydrologic Conditions in Georgia, 2013 the U.S
Return to Normal Streamflows and Water Levels: Summary of Hydrologic Conditions in Georgia, 2013 The U.S. Geological Survey (USGS) emphasize the need for accurate, timely data Water Resources Internet Tools South Atlantic Water Science Center (SAWSC) to help Federal, State, and local officials make Georgia office, in cooperation with local, informed decisions regarding the management Historically, hydrologic data collected State, and other Federal agencies, maintains and conservation of Georgia’s water resources by the USGS were compiled into annual data a long-term hydrologic monitoring network for agricultural, recreational, ecological, and reports; however, this method of publication of more than 340 real-time continuous-record water-supply needs and for use in protecting has been discontinued. Current and historical streamflow-gaging stations (streamgages), life and property. data are now available through the National including 10 real-time lake-level monitoring Drought conditions, persistent in the area Water Information System Web interface, or stations, 67 real-time surface-water-quality since 2010, continued into the 2013 WY. In NWISWeb, at http://waterdata.usgs.gov/nwis/ monitors, and several water-quality sampling February 2013, Georgia was free of extreme (U.S. Geological Survey, 2013a). programs. Additionally, the SAWSC Georgia (D3) drought conditions, as defined by the The USGS has several water resources office operates more than 180 groundwater U.S. Drought Monitor, for the first time Internet tools designed to provide users with monitoring wells, 39 of which are real-time. The since August 2010 due to extended periods current streamflow and groundwater data, wide-ranging coverage of streamflow, reservoir, of heavy rainfall (U.S. -
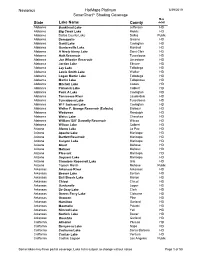
Navionics Hotmaps Platinum Sonarcharttm Shading Coverage
Navionics HotMaps Platinum 5/29/2019 SonarChartTM Shading Coverage Map State Lake Name County detail Alabama Bankhead Lake Jefferson HD Alabama Big Creek Lake Mobile HD Alabama Dallas County Lake Dallas Public Alabama Demopolis Greene HD Alabama Gantt Lake Covington HD Alabama Guntersville Lake Marshall HD Alabama H Neely Henry Lake Saint Clair HD Alabama Holt Reservoir Tuscaloosa HD Alabama Joe Wheeler Reservoir Limestone HD Alabama Jordan Lake Elmore HD Alabama Lay Lake Talladega HD Alabama Lewis Smith Lake Walker HD Alabama Logan Martin Lake Talladega HD Alabama Martin Lake Tallapoosa HD Alabama Mitchell Lake Coosa HD Alabama Pickwick Lake Colbert HD Alabama Point A Lake Covington HD Alabama Tennessee River Lauderdale HD Alabama Tuscaloosa Lake Tuscaloosa HD Alabama W F Jackson Lake Covington HD Alabama Walter F. George Reservoir (Eufaula) Barbour HD Alabama Wedowee Randolph HD Alabama Weiss Lake Cherokee HD Alabama William 'Bill' Dannelly Reservoir Wilcox HD Alabama Wilson Lake Colbert HD Arizona Alamo Lake La Paz HD Arizona Apache Lake Maricopa HD Arizona Bartlett Reservoir Maricopa HD Arizona Canyon Lake Maricopa HD Arizona Mead Mohave HD Arizona Mohave Mohave HD Arizona Pleasant Maricopa HD Arizona Saguaro Lake Maricopa HD Arizona Theodore Roosevelt Lake Gila HD Arizona Topock Marsh Mohave Public Arkansas Arkansas River Arkansas HD Arkansas Beaver Lake Benton HD Arkansas Bull Shoals Lake Marion HD Arkansas Chicot Chicot HD Arkansas Dardanelle Logan HD Arkansas De Gray Lake Clark HD Arkansas Greers Ferry Lake Cleburne HD Arkansas Greeson Pike HD Arkansas Hamilton Garland HD Arkansas Maumelle Pulaski HD Arkansas Nimrod Lake Yell HD Arkansas Norfork Lake Baxter HD Arkansas Ouachita Garland HD California Almanor Plumas HD California Berryessa Lake Napa HD California Cachuma Lake Santa Barbara HD California Casitas Lake Ventura HD California Castaic Lagoon Los Angeles Public Some lakes may have partial coverage. -

June 29-July 5, 2014
Department of Natural Resources Law Enforcement Division Field Operations Weekly Report June 29-July 5, 2014 This report is a broad sampling of events that have taken place in the past week, but does not include all actions taken by the Law Enforcement Division. Region I- Calhoun (Northwest) MURRAY COUNTY On July 4th, Sgt. John Vanlandingham, Cpl Casey Jones and Cpl. James Keener made twelve vessel stops. The rangers addressed various violations including, fishing without license, skiing after sunset, insufficient PFD’s and improper registration. On July 5th, Cpl. Casey Jones and RFC Joe Hill made fifteen vessel stops and addressed several violations that included failure to obey regulatory marker, operating vessel without registration, fishing without a license, improper lights, violations of rules of the road, insufficient PFD’s and violation of the 100’ rule. On July 6th, Cpl. Casey Jones and Wildlife Tech. III Larry Etheridge stopped six vessels on Carters Lake and addressed several violations including, towing skier without observer, failure to obey regulatory marker and violation 100’ rule. On July 6th, Cpl. Casey Jones charged a Catoosa County woman for possession of marijuana less than one ounce. Region II- Gainesville (Northeast) DAWSON COUNTY On July 5th, Ranger Shane Brown patrolled Amicalola Falls State Park. Ranger Brown observed subjects fishing at the reflection pool. One subject didn’t have a fishing license or a trout stamp. The subject was issued one citation and one warning. LAKE LANIER (DAWSON, FORSYTH, GWINNETT, HALL, & LUMPKIN COUNTIES) On June 29th, Sgt. Lee Brown and Ranger Shane Brown patrolled the North end of Lake Lanier. -

2016 Alabama 303(D) List (TN5097)
2016 Alabama §303(d) List Assessment Unit ID Waterbody Name Type River Basin County Uses Causes Sources Size Unit Downstream / Upstream Year Priority Type Locations Listed AL03150201-0101-200 Callaway Creek R Alabama Elmore Fish & Wildlife Nutrients Agriculture 13.02 miles Bouldin tailrace canal / 2010 H Municipal its source AL03150201-0104-302 Three Mile Branch R Alabama Montgomery Fish & Wildlife Pathogens (E. coli) Urban development 7.65 miles Lower Wetumpka Road / 2010 L its source AL03150201-0104-302 Three Mile Branch R Alabama Montgomery Fish & Wildlife Pesticides (Dieldrin) Unknown source 7.65 miles Lower Wetumpka Road / 2002 L its source AL03150201-0104-302 Three Mile Branch R Alabama Montgomery Fish & Wildlife Siltation (habitat alteration) Urban development 7.65 miles Lower Wetumpka Road / 2010 L its source AL03150201-0105-300 Mill Creek R Alabama Autauga Fish & Wildlife Siltation (habitat alteration) Urban development 8.71 miles Still Creek / 2010 L Elmore its source AL03150201-1006-101 Mulberry Creek R Alabama Autauga Swimming Pathogens (E. coli) Pasture grazing 22.20 miles Alabama River / 2016 L Dallas Fish & Wildlife Harris Branch AL03150201-1207-301 Sixmile Creek R Alabama Dallas Fish & Wildlife Metals (Mercury) Atmospheric deposition 1.23 miles Alabama River / 2012 L Fourmile Creek AL03150203-0103-200 Coffee Creek R Alabama Dallas Fish & Wildlife Nutrients Pasture grazing 7.67 miles Tayloe Creek / 2010 L Perry its source AL03150203-0103-200 Coffee Creek R Alabama Dallas Fish & Wildlife Pathogens (E. coli) Pasture grazing -
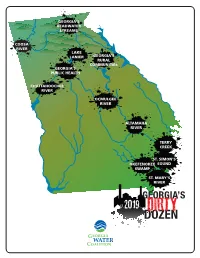
Dirty Dozen Report Because Pollution from the Mill Continues
GEORGIA’S HEADWATER STREAMS COOSA RIVER LAKE LANIER GEORGIA’S RURAL COMMUNITIES GEORGIA’S PUBLIC HEALTH CHATTAHOOCHEE RIVER OCMULGEE RIVER ALTAMAHA RIVER TERRY CREEK ST. SIMON’S OKEFENOKEE SOUND SWAMP ST. MARY’S RIVER 2019’s Worst Offenses Against GEORGIA’S WATER GEORGIA WATER COALITION’S DIRTY DOZEN A Call to Action The Georgia Water Coalition’s Dirty Dozen list highlights the politics, policies and issues that threaten the health of Georgia’s water and the well-being of 10 million Georgians. The purpose of the report is not to identify the state’s “most polluted places.” Instead the report is a call to action for Georgia’s leaders and its citizens to solve ongoing pollution problems, eliminate potential threats to Georgia’s water and correct state and federal policies and actions that lead to polluted water. Unfortunately, this year’s report includes seven issues that are making return visits to this inauspicious list. Topping that category is pollution of the Altamaha River from the Rayonier Advanced Materials (RAM) chemical pulp mill in Jesup. The facility is making a record seventh appearance in the Dirty Dozen report because pollution from the mill continues. Next year, Georgia’s Environmental Protection Division (EPD) will issue a new pollution control permit for the facility, but if EPD’s actions in recent years are any indication, it seems unlikely that this new permit will fix this ongoing pollution problem. The state agency has repeatedly defended the existing and weak The Georgia Water Coalition’s Dirty Dozen report is a pollution control permit and last year took the extraordinary step of call to action for Georgia’s leaders and citizens to solve changing state laws to make it easier for RAM to continue polluting ongoing pollution problems, eliminate potential threats Georgia’s largest river. -

A History of the Middle of Georgia 1
Jackson: A History of the Middle of Georgia 1 In the southwestern section of town is a place that was once howling grounds for two packs of wolves. The two packs, one from Yellow Water and one from Sandy Creek, made that area their nocturnal meeting area and made nights frightful to early settlers due to the hideous howling. Before Jackson was created, the area where the downtown now stands was only two Indian trails that crossed where a 10’ by 12’ log cabin stood and was used as a post office. The first hanging in Jackson took place before the city was created in the middle of what would become Third Street between the Furlow and Slaughter residences. Two White men were hanged there on an old chestnut tree and were buried in the backyard of the Furlow place. 1818 In 1818, the first church services were held in what would become the City of Jackson. They were conducted by a Methodist, Mrs. Mary Williams Buttrill, in a log house erected by her slaves at what is now the east entrance to the Jackson Cemetery. Buttrill died in 1830, but for years she took her own seven boys, three daughters, slaves and local children into this log house one afternoon a week to hold religious services using her Bible and prayer book. 1822 The Southern Railway was built through the area that would soon become Butts County in 1822. A Western Union telegraph service came to the area soon after that. 1825 Butts County was created by an act of the Georgia General Assembly on December 24, 1825. -

Extreme Drought: Summary of Hydrologic Conditions in Georgia
Water-Quality Monitoring in Water-quantity and quality information are Ogeechee River at Ga. 24, near Oliver, Ga. 02202190 Georgia in Cooperation with the equally important for insuring adequate water 4,000 Lakes and Reservoirs availability for human consumption, industrial uses, May 21, 2011, reported fish kill Major lakes and reservoirs Georgia Department of Natural and aquatic ecosystems. Streamflow conditions are Sample date 2/10/11 throughout Georgia are managed Lake SC Resources Environmental a primary driver of nonpoint-source-related water Discharge 2,750 ft3/s Sidney Hartwell Lake primarily by the U.S. Army Corps of Lanier quality and the most important component affecting WT 8.0 °C Protection Division (EPD) 3,000 Engineers and Georgia Power Company Richard B water quality in streams (Hirsch and others, 2006). SC 71 µg/L Weiss Russell Lake Extreme Drought: Summary of Hydrologic Conditions Allatoona TSS 9.5 mg/L to provide water for public and industrial Lake Lake The USGS, in cooperation with the The USGS – GaEPD discrete water-quality J Strom BacT-FC 170 MPN use, flood protection, power generation, Atlanta Thurmond Georgia Department of Natural Resources sampling program is designed to collect data in Georgia, 2011 wildlife management, and recreation. Lake Lake Environmental Protection Division (GaEPD), systematically, regardless of hydrologic conditions. West Sinclair collects nearly 1,000 monthly chemical and The graph (top right) shows the water-quality Managing lakes and reservoirs requires Point 2,000 Sample date 1/27/11 The United States Geological Survey flow for several days, including 02314500 U.S. Geological Survey, 2012a) were computer models that rely on USGS Lake nutrient samples and about 800 fecal coliform sampling efforts by the USGS at Ogeechee River 3 GEORGIA Discharge 689 ft /s (USGS) Georgia Water Science Center Suwanee River at U.S.