(Epi)Genotype–Phenotype Correlations in Beckwith–Wiedemann Syndrome
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Rare Pancreatic Tumors
Published online: 2020-04-29 THIEME 64 ReviewRare Pancreatic Article Tumors Choudhari et al. Rare Pancreatic Tumors Amitkumar Choudhari1,2 Pooja Kembhavi1,2 Mukta Ramadwar3,4 Aparna Katdare1,2 Vasundhara Smriti1,2 Akshay D. Baheti1,2 1Department of Radiodiagnosis, Tata Memorial Hospital, Mumbai, Address for correspondence Akshay D. Baheti, MD, Department of Maharashtra, India Radiodiagnosis, Tata Memorial Hospital, Ernest , Borges Marg Parel 2Department of Radiodiagnosis, Homi Bhabha National University, Mumbai 400012, India (e-mail: [email protected]). Mumbai, Maharashtra, India 3Department of Pathology, Tata Memorial Hospital, Mumbai, Maharashtra, India 4Department of Pathology, Homi Bhabha National University, Mumbai, Maharashtra, India J Gastrointestinal Abdominal Radiol ISGAR 2020;3:64–74 Abstract Pancreatic ductal adenocarcinoma, neuroendocrine tumor, and cystic pancreatic neo- plasms are the common pancreatic tumors most radiologists are familiar with. In this Keywords article we review the clinical presentation, pathophysiology, and radiology of rare pan- ► pancreatic cancer creatic neoplasms. While the imaging features are usually nonspecific and diagnosis is ► uncommon based on pathology, the radiology along with patient demographics, history, and lab- ► pancreatoblastoma oratory parameters can often help indicate the diagnosis of an uncommon pancreatic ► acinar cell neoplasm and guide appropriate management in these cases. ► lymphoma Introduction hyperlipasemia may rarely lead to extraabdominal manifes- tations like ectopic subcutaneous fat necrosis and polyarthri- Pancreatic tumors of various histological subtypes can be tis (lipase hypersecretion syndrome).4 encountered in clinical practice, most common being pan- These tumors are hypoenhancing compared with the pan- creatic ductal adenocarcinoma (PDAC), which constitutes creas and are frequently associated with cystic or necrotic 85% of all pancreatic neoplasms.1 Histologically pancreat- areas as well as calcifications5,6 (►Fig. -
Solid Tumour Section Mini Review
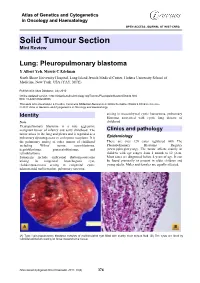
Atlas of Genetics and Cytogenetics in Oncology and Haematology OPEN ACCESS JOURNAL AT INIST-CNRS Solid Tumour Section Mini Review Lung: Pleuropulmonary blastoma Y Albert Yeh, Morris C Edelman North Shore University Hospital, Long Island Jewish Medical Center, Hofstra University School of Medicine, New York, USA (YAY, MCE) Published in Atlas Database: July 2010 Online updated version : http://AtlasGeneticsOncology.org/Tumors/PleuropulmblastomID6040.html DOI: 10.4267/2042/45006 This work is licensed under a Creative Commons Attribution-Noncommercial-No Derivative Works 2.0 France Licence. © 2011 Atlas of Genetics and Cytogenetics in Oncology and Haematology arising in mesenchymal cystic hamartoma, pulmonary Identity blastoma associated with cystic lung disease of Note childhood. Pleuropulmonary blastoma is a rare aggressive malignant tumor of infancy and early childhood. The Clinics and pathology tumor arises in the lung and pleura and is regarded as a pulmonary dysontogenetic or embryonic neoplasm. It is Epidemiology the pulmonary analog of other tumors of childhood There are over 120 cases registered with The including Wilms' tumor, neuroblastoma, Pleuropulmonary Blastoma Registry hepatoblastoma, pancreatoblastoma, and (www.ppbregistry.org). The tumor affects mainly in retinoblastoma. children with age ranges from 1 month to 12 years. Synonyms include embryonal rhabomyosarcoma Most cases are diagnosed before 4 years of age. It can arising in congenital bronchogenic cyst, be found prenatally or present in older children and rhabdomyosarcoma arising in congenital cystic young adults. Males and females are equally affected. adenomatoid malformation, pulmonary sarcoma (A) Type I pleuropulmonary blastoma consists of multiloculated cyst filled with scanty clear serous fluid. (B) The cysts are lined by cuboidal epithelium resting on loose mesenchymal tissue. -

Input Data Dictionary Statistics Canada Health Statistics Division
Component of Statistics Canada Catalogue no. 82-225-XIE2005000 ISSN: 1715-2100 O Canadian Cancer Registry Manual 2005 Input Data Dictionary Statistics Canada Health Statistics Division Canadian Cancer Registry Input Data Dictionary Published by authority of the Minister responsible for Statistics Canada © Minister of Industry, 2003 Material appearing in this publication may be reproduced or copied without permission; however, the following citation to indicate the source must be used: "Data sources: Statistics Canada, Canadian Cancer Registry, Ottawa, 2003." February 2005 Catalogue no. 84-601-XIE Frequency: Irregular Ottawa La version française de cette publication est disponible gratuitement sur le site Internet de Statistique Canada (no 84-601-XIF au catalogue). Note of Appreciation Canada owes the success of its statistical system to a long-standing partnership between Statistics Canada, the citizens of Canada, its businesses, governments and other institutions. Accurate and timely statistical information could not be produced without their continued cooperation and goodwill. Canadian Cancer Registry – Input Data Dictionary Table of Contents Page 1.0 Introduction........................................................................................................................1 1.1 Content of Input Data Dictionary ............................................................................1 1.2 CCR Overview.........................................................................................................2 1.2.1 Canadian Cancer -

Download Download
Doi: 10.32677/IJCH.2016.v03.i04.021 Case Report Pain abdomen in a child - An uncommon cause Varun Alwadhi, Aashima Dabas, Anju Aggarwal, M M A Faridi From Department of Pediatrics, University College of Medical Sciences and Guru Tegh Bahadur Hospital, New Delhi, India Correspondence to: Dr. Anju Aggarwal, Flat No. 3C Block, C2B Janakpuri, New Delhi - 110 058, India. Phone: +91-9910329791. E-mail: [email protected] Received – 16 August 2016 Initial Review – 18 September 2016 Published Online – 24 September 2016 ABSTRACT Diagnosis, identification of underlying etiology and management of pain abdomen, remains difficult. Tumors presenting as abdominal pain are rare in children. We report a case of 11-year old boy presenting with pain abdomen. On examination, he had a lump in left hypochondrium. Gastrointestinal tumors constitute about 12% of abdominal masses, 2% of which are pancreatic tumors. He underwent laparotomy was diagnosed as desmoplastic small round cell tumor in the pancreas. This report presents an uncommon cause of a common pediatric problem. Key words: Desmoplastic small round cell tumor, Diagnosis, Outcomes, Pancreas bdominal pain is a common problem in pediatric (Fig. 1). Contrast-enhanced computed tomography of thorax did practice. Common causes of abdominal pain include not reveal any metastasis. Bone marrow aspiration was normal. The Aenteritis, infection, worm infestation, constipation, patient underwent open laparotomy with resection of pancreatic food allergy, peptic ulcer, and obstruction. Tumors presenting body and tail and splenectomy and removal of adjoining lymph as abdominal pain are rare in children. Most common tumor nodes (Fig. 2). Post-operative histopathology findings confirmed masses in childhood are neuroblastoma, Wilm’s tumors, non- the diagnosis of DSRCT of the pancreas (Grade 4-undifferentiated, Hodgkin’s lymphoma, germ cell tumor, and hepatoblastoma [1]. -

New Jersey State Cancer Registry List of Reportable Diseases and Conditions Effective Date March 10, 2011; Revised March 2019
New Jersey State Cancer Registry List of reportable diseases and conditions Effective date March 10, 2011; Revised March 2019 General Rules for Reportability (a) If a diagnosis includes any of the following words, every New Jersey health care facility, physician, dentist, other health care provider or independent clinical laboratory shall report the case to the Department in accordance with the provisions of N.J.A.C. 8:57A. Cancer; Carcinoma; Adenocarcinoma; Carcinoid tumor; Leukemia; Lymphoma; Malignant; and/or Sarcoma (b) Every New Jersey health care facility, physician, dentist, other health care provider or independent clinical laboratory shall report any case having a diagnosis listed at (g) below and which contains any of the following terms in the final diagnosis to the Department in accordance with the provisions of N.J.A.C. 8:57A. Apparent(ly); Appears; Compatible/Compatible with; Consistent with; Favors; Malignant appearing; Most likely; Presumed; Probable; Suspect(ed); Suspicious (for); and/or Typical (of) (c) Basal cell carcinomas and squamous cell carcinomas of the skin are NOT reportable, except when they are diagnosed in the labia, clitoris, vulva, prepuce, penis or scrotum. (d) Carcinoma in situ of the cervix and/or cervical squamous intraepithelial neoplasia III (CIN III) are NOT reportable. (e) Insofar as soft tissue tumors can arise in almost any body site, the primary site of the soft tissue tumor shall also be examined for any questionable neoplasm. NJSCR REPORTABILITY LIST – 2019 1 (f) If any uncertainty regarding the reporting of a particular case exists, the health care facility, physician, dentist, other health care provider or independent clinical laboratory shall contact the Department for guidance at (609) 633‐0500 or view information on the following website http://www.nj.gov/health/ces/njscr.shtml. -

Pancreatoblastoma: a Rare Tumour Accidentally Found Naik V R, Jaafar H, Leow V M, Bhavaraju V M K
Case Report SingaporeSingapore Med Med J 2006; J 2006; 47(3) 47(3) : 232 : 1 Pancreatoblastoma: a rare tumour accidentally found Naik V R, Jaafar H, Leow V M, Bhavaraju V M K ABSTRACT raising the possibility that genetic events on A 15-year-old girl, who was previously well, chromosome 11p might play a role. complained of a mass in the abdomen after The clinical presentations of these tumours a minor motor vehicle accident. Physical are varied. They can present as abdominal pain, and radiological investigations revealed a abdominal mass, diarrhoea, or upper gastrointestinal mass in the body of pancreas containing bleeding. Most of the time, they are asymptomatic. proteinaceous material and multiple The presenting features are highly non-specific and nodules in both lobes of liver. Serological this leads to diagnostic dilemmas. The tumour is investigations for malignancy were normal. slightly more frequent in males, with the median Histopathological examination of the resected age of presentation being five years. Though specimen showed pancreatoblastoma. malignant, these tumours have an indolent course. Pancreatoblastoma is an unusual malignant They can be cured by complete resection alone tumour seen in infants and children although and in cases of unresectable tumours, incomplete rare cases have also been reported in adults. resection and in those with metastatic lesions, They are clinicopathologically distinct from radiotherapy or chemotherapy may be given. The adult pancreatic ductal carcinoma. The prognosis is worse in the presence of synchronous histogenesis, clinical features and treatment or metachronous metastasis and non-resectable options are discussed along with presentation (1) Department of disease at presentation . -

TCF-001 TRACK (Target Rare Cancer Knowledge)
TCF-001 TRACK (Target Rare Cancer Knowledge) Cancers not listed here may be enrolled with the approval of the Principal Investigator on a case by case basis. Tier Tumor 1 EPITHELIAL TUMORS OF CERVIX UTERI 2 Squamous cell carcinoma with variants of cervix uteri 3 Squamous carcinoma 3 Squamous cell carcinoma nonkeratinizing, NOS 3 Squamous cell carcinoma keratinizing, NOS 3 Papillary squamous cell carcinoma 3 Papillary carcinoma, NOS 3 Verrucous/Warty carcinoma 3 Basaloid carcinoma 3 Squamous cell carcinoma spindle cell 3 Lymphoepithelial carcinoma 3 Transitional cell carcinoma, NOS 3 Glassy cell carcinoma 2 Adenocarcinoma with variants of cervix uteri 3 Adenocarcinoma, NOS 3 Adenocarcinoma with squamous metaplasia 3 Mucinous adenocarcinoma 3 Clear cell adenocarcinoma, NOS 3 Endometrioid adenocarcinoma, NOS 3 Serous cystadenocarcinoma, NOS 3 Signet ring cell carcinoma 3 Mesonephroma malignant 3 Villous adenocarcinoma 3 Mucinous adenocarcinoma, endocervical type 3 Adenocarcinoma intestinal type 3 Mixed cell adenocarcinoma 2 UnDifferentiateD carcinoma of cervix uteri 1 MIXED EPITHELIAL AND MESENCHYMAL TUMORS OF UTERUS 3 Mullerian mixed tumor 3 Adenosarcoma 1 EPITHELIAL TUMORS OF NASAL CAVITY AND SINUSES 2 Squamous cell carcinoma with variants of nasal cavity and sinuses 3 Squamous carcinoma 3 Verrucous carcinoma 3 Squamous cell carcinoma spindle cell 3 Papillary squamous cell carcinoma 3 Adenosquamous carcinoma Tier Tumor 3 Squamous cell carcinoma, adenoid 3 Basaloid squamous cell carcinoma 2 Lymphoepithelial carcinoma of nasal cavity and -

WHO Classification of Tumors of the Central Nervous System
Appendix A: WHO Classification of Tumors of the Central Nervous System WHO Classification 1979 • Ganglioneuroblastoma • Anaplastic [malignant] gangliocytoma and Zülch KJ (1979) Histological typing of tumours ganglioglioma of the central nervous system. 1st ed. World • Neuroblastoma Health Organization, Geneva • Poorly differentiated and embryonal tumours • Glioblastoma Tumours of Neuroepithelial tissue –– Variants: • Astrocytic tumours –– Glioblastoma with sarcomatous compo- • Astrocytoma nent [mixed glioblastoma and sarcoma] –– fibrillary –– Giant cell glioblastoma –– protoplasmic • Medulloblastoma –– gemistocytic –– Variants: • Pilocytic astrocytoma –– desmoplastic medulloblastoma • Subependymal giant cell astrocytoma [ven- –– medullomyoblastoma tricular tumour of tuberous sclerosis] • Medulloepithelioma • Astroblastoma • Primitive polar spongioblastoma • Anaplastic [malignant] astrocytoma • Gliomatosis cerebri • Oligodendroglial tumours • Oligodendroglioma Tumours of nerve sheath cells • Mixed-oligo-astrocytoma • Neurilemmoma [Schwannoma, neurinoma] • Anaplastic [malignant] oligodendroglioma • Anaplastic [malignant] neurilemmoma [schwan- • Ependymal and choroid plexus tumours noma, neurinoma] • Ependymoma • Neurofibroma –– Myxopapillary • Anaplastic [malignant]neurofibroma [neurofi- –– Papillary brosarcoma, neurogenic sarcoma] –– Subependymoma • Anaplastic [malignant] ependymoma Tumours of meningeal and related tissues • Choroid plexus papilloma • Meningioma • Anaplastic [malignant] choroid plexus papil- –– meningotheliomatous [endotheliomatous, -

Pancreatoblastoma: Cytologic and Histologic Analysis of 12 Cases Reveals Helpful Criteria in Their Diagnosis and Distinction from Common Mimics
DR. MICHELLE DIAN REID (Orcid ID : 0000-0002-3812-4098) DR. CARLIE S SIGEL (Orcid ID : 0000-0001-6276-3968) DR. ANJALI SAQI (Orcid ID : 0000-0003-3133-9886) Article type : Original Article Pancreatoblastoma: Cytologic and Histologic Analysis of 12 Cases Reveals Helpful Criteria in Their Diagnosis and Distinction from Common Mimics Michelle D Reid1, Shristi Bhattarai2, Rondell P Graham3, Burcin Pehlivanoglu1, Carlie S Sigel4, Jiaqi Shi5, Anjali Saqi6, Maryam Shirazi6, Yue Xue1, Olca Basturk4, Volkan Adsay7 1Department of Pathology, Emory University Hospital, Atlanta GA; 2Department of Biology, Georgia State University, Atlanta GA; Departments of Pathology, 3Mayo Clinic, Rochester, MN, 4Memorial Sloan Kettering Cancer Center, New York, NY, 5University of Michigan, Ann Harbor, MI, 6Columbia University Medical Center, New York, NY and 7Koç University Hospital, Istanbul, Turkey Author contribution statement: Michelle Reid, Volkan Adsay: conceptualization, data curation, formal analysis, investigation, methodology, resources, supervision, writing, editing original draft. Shristi Bhattarai, Burcin Pehlivanoglu: data curation, formal analysis, investigation, methodology, project administration. Rondell Graham, Carlie Sigel, Jiaqi Shi, Maryam Shirazi, Yue Xue, Olca Basturk: data curation, formal analysis, investigation, methodology, review and editing manuscript. Author Manuscript Running title: Cytologic diagnosis of pancreatoblastoma This is the author manuscript accepted for publication and has undergone full peer review but has not been through -

Rare Childhood Cancers
RARE CHILDHOOD CANCERS Robert Raphael, MD Associate Director, Survivors of Childhood Cancer Program UCSF Benioff Children’s Hospital Oakland Disclosures • No significant financial interests to disclose • Off-label use of medications discussed • Special thanks to Carlos Rodriguez-Galindo, MD Rare Cancers • General cancer incidence: 1.7 million cases/year in US • Rare disease: < 200,000 cases/year (US Orphan Drug Act of 1983) • Rare cancer: • < 15 per 100,000/year (National Cancer Institute 2004) • < 6 per 100,000/year (European RARECARE consortium 2011) • 181 cancers meet RARECARE definition in US • 119 are very rare (≤ 0.5 per 100,000): 3% of all cancers • Overall 20% of cancers in US considered rare • Rare cancers are not so rare CA Cancer J Clin 2017; 67; 261-272 Rare Cancers CA Cancer J Clin 2017; 67; 261-272 Rare Cancers • Unique challenges of studying rare cancers: • Difficult to conduct clinical research • Logistics of identifying and enrolling patients • Lack of funding and awareness • Barriers to collaborative/international trials • Paucity of basic research • Limited biologic specimens • Few resources devoted to uncommon diseases • Delays in recognition and diagnosis • Limited treatment options due to lack of evidence • 59% of rare tumors advanced stage at diagnosis (vs 45%) • Decreased survival rates for rare vs common cancers Rare Cancers Survival: rare vs common (by age) CA Cancer J Clin 2017; 67; 261-272 Childhood Cancer • 1,688,780 new cancers diagnosed in US in 2017 • Only 15,270 in children <20 years old • Less than 1% -

Rare Tumors and Lesions of the Pancreas
Rare Tumors and Lesions of the Pancreas John A. Stauffer, MD, Horacio J. Asbun, MD* KEYWORDS Pancreatectomy Pancreatic neoplasm Anaplastic carcinoma Adenosquamous carcinoma Solid pseudopapillary tumor Acinar cell carcinoma Primary pancreatic lymphoma Unusual pancreas tumors KEY POINTS Rare pancreatic tumors of the pancreas include adenocarcinoma variants, such as anaplastic carcinoma, adenosquamous carcinoma, colloid, hepatoid, and medullary carcinoma. Other neoplasms include acinar cell carcinoma, solid pseudopapillary tumor, sarcomas, or lymphomas. Benign solid or cystic masses, such as hamartoma, hemangioma, lymphangioma, or others also may mimic neoplastic disease. The pancreas may be the site of isolated metastatic disease, such as renal cell cancer, colorectal cancer, melanoma, and other carcinomas. Pancreatic inflammatory diseases may mimic solid neoplasms of the pancreas. Primary pancreatic ductal adenocarcinoma (PDAC) is the most common neoplasm of the pancreas. Pancreatic neuroendocrine tumors (PNETs) are much less common but their incidence has increased over the past decade due to the increased use of cross- sectional imaging.1 Cystic lesions, such as intraductal papillary mucinous neoplasm (IPMN), mucinous cystic neoplasms (MCN), and serous cystic neoplasms (SCN) are also relatively common. The pancreas is a complex organ that harbors a wide array of diseases. There are a variety of non-neoplastic conditions that mimic PDAC, such as groove pancreatitis (GP) and autoimmune pancreatitis (AIP).2,3 Additionally, there are a handful of other rare neoplastic lesions infrequently found in patients with pancreatic masses that range from well known (eg, solid pseudopapillary neoplasm and acinar cell carcinoma) to less well known (eg, leiomyosarcoma and hepatoid carcinoma). Rare cystic lesions can be misdiagnosed for the more common Disclosures: The authors have nothing to disclose. -

NYS Cancer Registry Facility Reporting Manual
The New York State CANCER REGISTRY Facility Reporting Manual 2021 - EDITION THE NEW YORK STATE DEPARTMENT OF HEALTH STATE OF NEW YORK KATHY HOCHUL, GOVERNOR DEPARTMENT OF HEALTH HOWARD A. ZUCKER, M.D., J.D., COMMISSIONER The NYSCR Reporting Manual Revised September 2021 New York State Cancer Registry Reporting Manual Table of Contents ACKNOWLEDGEMENT PART ONE – OVERVIEW PART TWO – CONFIDENTIALITY PART THREE - REPORTABLE CONDITIONS AND TERMINOLOGY PART FOUR - DATA ITEMS AND DESCRIPTIONS PART FIVE - CASEFINDING PART SIX - DEATH CERTIFICATE ONLY AND DEATH CLEARANCE LISTS PART SEVEN – QUALITY ASSURANCE PART EIGHT – ELECTRONIC REPORTING APPENDIX A - NYS PUBLIC HEALTH LAW APPENDIX B – HIPAA INFORMATION The NYSCR Reporting Manual – Table of Contents Revised September 2021 Page Left Blank Intentionally The NYSCR Reporting Manual Revised September 2021 ACKNOWLEDGEMENT We wish to acknowledge the Centers for Disease Control and Prevention's (CDC) National Program of Cancer Registries (NPCR) and the National Cancer Institute’s (NCI) Surveillance Epidemiology and End Results program (SEER) for their support. Production of this Reporting Manual was supported in part by a cooperative agreement awarded to the New York State Department of Health by the NPCR and a contract with SEER. Its contents are solely the responsibility of the New York State Department of Health and do not necessarily represent the official views of the CDC or NCI. The NYSCR Reporting Manual - Acknowledgement Revised September 2021 Page Left Blank Intentionally The NYSCR Reporting Manual Revised September 2021 New York State Cancer Registry Reporting Manual Part One – Overview 1.1 WHAT IS THE NEW YORK STATE CANCER REGISTRY? .................................... 1 1.2 WHY REPORT TO THE NYSCR? ..........................................................................