Pleuropulmonary Blastoma: Case Report
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Understanding Your Pathology Report: Benign Breast Conditions
cancer.org | 1.800.227.2345 Understanding Your Pathology Report: Benign Breast Conditions When your breast was biopsied, the samples taken were studied under the microscope by a specialized doctor with many years of training called a pathologist. The pathologist sends your doctor a report that gives a diagnosis for each sample taken. Information in this report will be used to help manage your care. The questions and answers that follow are meant to help you understand medical language you might find in the pathology report from a breast biopsy1, such as a needle biopsy or an excision biopsy. In a needle biopsy, a hollow needle is used to remove a sample of an abnormal area. An excision biopsy removes the entire abnormal area, often with some of the surrounding normal tissue. An excision biopsy is much like a type of breast-conserving surgery2 called a lumpectomy. What does it mean if my report uses any of the following terms: adenosis, sclerosing adenosis, apocrine metaplasia, cysts, columnar cell change, columnar cell hyperplasia, collagenous spherulosis, duct ectasia, columnar alteration with prominent apical snouts and secretions (CAPSS), papillomatosis, or fibrocystic changes? All of these are terms that describe benign (non-cancerous) changes that the pathologist might see under the microscope. They do not need to be treated. They are of no concern when found along with cancer. More information about many of these can be found in Non-Cancerous Breast Conditions3. What does it mean if my report says fat necrosis? Fat necrosis is a benign condition that is not linked to cancer risk. -

Rare Pancreatic Tumors
Published online: 2020-04-29 THIEME 64 ReviewRare Pancreatic Article Tumors Choudhari et al. Rare Pancreatic Tumors Amitkumar Choudhari1,2 Pooja Kembhavi1,2 Mukta Ramadwar3,4 Aparna Katdare1,2 Vasundhara Smriti1,2 Akshay D. Baheti1,2 1Department of Radiodiagnosis, Tata Memorial Hospital, Mumbai, Address for correspondence Akshay D. Baheti, MD, Department of Maharashtra, India Radiodiagnosis, Tata Memorial Hospital, Ernest , Borges Marg Parel 2Department of Radiodiagnosis, Homi Bhabha National University, Mumbai 400012, India (e-mail: [email protected]). Mumbai, Maharashtra, India 3Department of Pathology, Tata Memorial Hospital, Mumbai, Maharashtra, India 4Department of Pathology, Homi Bhabha National University, Mumbai, Maharashtra, India J Gastrointestinal Abdominal Radiol ISGAR 2020;3:64–74 Abstract Pancreatic ductal adenocarcinoma, neuroendocrine tumor, and cystic pancreatic neo- plasms are the common pancreatic tumors most radiologists are familiar with. In this Keywords article we review the clinical presentation, pathophysiology, and radiology of rare pan- ► pancreatic cancer creatic neoplasms. While the imaging features are usually nonspecific and diagnosis is ► uncommon based on pathology, the radiology along with patient demographics, history, and lab- ► pancreatoblastoma oratory parameters can often help indicate the diagnosis of an uncommon pancreatic ► acinar cell neoplasm and guide appropriate management in these cases. ► lymphoma Introduction hyperlipasemia may rarely lead to extraabdominal manifes- tations like ectopic subcutaneous fat necrosis and polyarthri- Pancreatic tumors of various histological subtypes can be tis (lipase hypersecretion syndrome).4 encountered in clinical practice, most common being pan- These tumors are hypoenhancing compared with the pan- creatic ductal adenocarcinoma (PDAC), which constitutes creas and are frequently associated with cystic or necrotic 85% of all pancreatic neoplasms.1 Histologically pancreat- areas as well as calcifications5,6 (►Fig. -
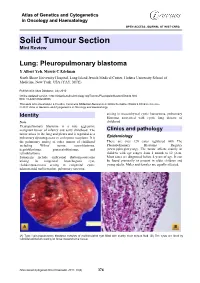
Solid Tumour Section Mini Review
Atlas of Genetics and Cytogenetics in Oncology and Haematology OPEN ACCESS JOURNAL AT INIST-CNRS Solid Tumour Section Mini Review Lung: Pleuropulmonary blastoma Y Albert Yeh, Morris C Edelman North Shore University Hospital, Long Island Jewish Medical Center, Hofstra University School of Medicine, New York, USA (YAY, MCE) Published in Atlas Database: July 2010 Online updated version : http://AtlasGeneticsOncology.org/Tumors/PleuropulmblastomID6040.html DOI: 10.4267/2042/45006 This work is licensed under a Creative Commons Attribution-Noncommercial-No Derivative Works 2.0 France Licence. © 2011 Atlas of Genetics and Cytogenetics in Oncology and Haematology arising in mesenchymal cystic hamartoma, pulmonary Identity blastoma associated with cystic lung disease of Note childhood. Pleuropulmonary blastoma is a rare aggressive malignant tumor of infancy and early childhood. The Clinics and pathology tumor arises in the lung and pleura and is regarded as a pulmonary dysontogenetic or embryonic neoplasm. It is Epidemiology the pulmonary analog of other tumors of childhood There are over 120 cases registered with The including Wilms' tumor, neuroblastoma, Pleuropulmonary Blastoma Registry hepatoblastoma, pancreatoblastoma, and (www.ppbregistry.org). The tumor affects mainly in retinoblastoma. children with age ranges from 1 month to 12 years. Synonyms include embryonal rhabomyosarcoma Most cases are diagnosed before 4 years of age. It can arising in congenital bronchogenic cyst, be found prenatally or present in older children and rhabdomyosarcoma arising in congenital cystic young adults. Males and females are equally affected. adenomatoid malformation, pulmonary sarcoma (A) Type I pleuropulmonary blastoma consists of multiloculated cyst filled with scanty clear serous fluid. (B) The cysts are lined by cuboidal epithelium resting on loose mesenchymal tissue. -

Input Data Dictionary Statistics Canada Health Statistics Division
Component of Statistics Canada Catalogue no. 82-225-XIE2005000 ISSN: 1715-2100 O Canadian Cancer Registry Manual 2005 Input Data Dictionary Statistics Canada Health Statistics Division Canadian Cancer Registry Input Data Dictionary Published by authority of the Minister responsible for Statistics Canada © Minister of Industry, 2003 Material appearing in this publication may be reproduced or copied without permission; however, the following citation to indicate the source must be used: "Data sources: Statistics Canada, Canadian Cancer Registry, Ottawa, 2003." February 2005 Catalogue no. 84-601-XIE Frequency: Irregular Ottawa La version française de cette publication est disponible gratuitement sur le site Internet de Statistique Canada (no 84-601-XIF au catalogue). Note of Appreciation Canada owes the success of its statistical system to a long-standing partnership between Statistics Canada, the citizens of Canada, its businesses, governments and other institutions. Accurate and timely statistical information could not be produced without their continued cooperation and goodwill. Canadian Cancer Registry – Input Data Dictionary Table of Contents Page 1.0 Introduction........................................................................................................................1 1.1 Content of Input Data Dictionary ............................................................................1 1.2 CCR Overview.........................................................................................................2 1.2.1 Canadian Cancer -

Download Download
Doi: 10.32677/IJCH.2016.v03.i04.021 Case Report Pain abdomen in a child - An uncommon cause Varun Alwadhi, Aashima Dabas, Anju Aggarwal, M M A Faridi From Department of Pediatrics, University College of Medical Sciences and Guru Tegh Bahadur Hospital, New Delhi, India Correspondence to: Dr. Anju Aggarwal, Flat No. 3C Block, C2B Janakpuri, New Delhi - 110 058, India. Phone: +91-9910329791. E-mail: [email protected] Received – 16 August 2016 Initial Review – 18 September 2016 Published Online – 24 September 2016 ABSTRACT Diagnosis, identification of underlying etiology and management of pain abdomen, remains difficult. Tumors presenting as abdominal pain are rare in children. We report a case of 11-year old boy presenting with pain abdomen. On examination, he had a lump in left hypochondrium. Gastrointestinal tumors constitute about 12% of abdominal masses, 2% of which are pancreatic tumors. He underwent laparotomy was diagnosed as desmoplastic small round cell tumor in the pancreas. This report presents an uncommon cause of a common pediatric problem. Key words: Desmoplastic small round cell tumor, Diagnosis, Outcomes, Pancreas bdominal pain is a common problem in pediatric (Fig. 1). Contrast-enhanced computed tomography of thorax did practice. Common causes of abdominal pain include not reveal any metastasis. Bone marrow aspiration was normal. The Aenteritis, infection, worm infestation, constipation, patient underwent open laparotomy with resection of pancreatic food allergy, peptic ulcer, and obstruction. Tumors presenting body and tail and splenectomy and removal of adjoining lymph as abdominal pain are rare in children. Most common tumor nodes (Fig. 2). Post-operative histopathology findings confirmed masses in childhood are neuroblastoma, Wilm’s tumors, non- the diagnosis of DSRCT of the pancreas (Grade 4-undifferentiated, Hodgkin’s lymphoma, germ cell tumor, and hepatoblastoma [1]. -

Intraoral Lipoma: a Case Report
Hindawi Publishing Corporation Case Reports in Medicine Volume 2014, Article ID 480130, 4 pages http://dx.doi.org/10.1155/2014/480130 Case Report Intraoral Lipoma: A Case Report L. K. Surej Kumar, Nikhil Mathew Kurien, Varun B. Raghavan, P. Varun Menon, and Sherin A. Khalam Department of Oral & Maxillofacial Surgery, PMS College of Dental Science & Research, Golden Hills, Vattappara, Venkode, Thiruvananthapuram 695028, India Correspondence should be addressed to P. Varun Menon; [email protected] Received 13 September 2013; Accepted 19 December 2013; Published 30 January 2014 Academic Editor: David W. Eisele Copyright © 2014 L. K. Surej Kumar et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Lipomas are rare in oral and maxillofacial regions although they are the most common tumours of mesenchymal origin in human body. The etiology remains unclear. Various different theories explain the pathogenesis of this adipose tissue tumour andalso different histological variants of oral lipoma have been given in literature. A case of intraoral lipoma occurring in mental region in a 77-year-old male is reported along with review of the literature. Wide surgical excision was performed and two-year followup showed excellent healing without any recurrence. Lipomas are benign soft tissue neoplasm of mature adipose tissue seen as a common entity in the head and neck region. Intraoral lipomas are a rare entity which may be noticed only during routine dental examinations. Most of them rarely cause pain, resulting in delay to seek treatment. -

New Jersey State Cancer Registry List of Reportable Diseases and Conditions Effective Date March 10, 2011; Revised March 2019
New Jersey State Cancer Registry List of reportable diseases and conditions Effective date March 10, 2011; Revised March 2019 General Rules for Reportability (a) If a diagnosis includes any of the following words, every New Jersey health care facility, physician, dentist, other health care provider or independent clinical laboratory shall report the case to the Department in accordance with the provisions of N.J.A.C. 8:57A. Cancer; Carcinoma; Adenocarcinoma; Carcinoid tumor; Leukemia; Lymphoma; Malignant; and/or Sarcoma (b) Every New Jersey health care facility, physician, dentist, other health care provider or independent clinical laboratory shall report any case having a diagnosis listed at (g) below and which contains any of the following terms in the final diagnosis to the Department in accordance with the provisions of N.J.A.C. 8:57A. Apparent(ly); Appears; Compatible/Compatible with; Consistent with; Favors; Malignant appearing; Most likely; Presumed; Probable; Suspect(ed); Suspicious (for); and/or Typical (of) (c) Basal cell carcinomas and squamous cell carcinomas of the skin are NOT reportable, except when they are diagnosed in the labia, clitoris, vulva, prepuce, penis or scrotum. (d) Carcinoma in situ of the cervix and/or cervical squamous intraepithelial neoplasia III (CIN III) are NOT reportable. (e) Insofar as soft tissue tumors can arise in almost any body site, the primary site of the soft tissue tumor shall also be examined for any questionable neoplasm. NJSCR REPORTABILITY LIST – 2019 1 (f) If any uncertainty regarding the reporting of a particular case exists, the health care facility, physician, dentist, other health care provider or independent clinical laboratory shall contact the Department for guidance at (609) 633‐0500 or view information on the following website http://www.nj.gov/health/ces/njscr.shtml. -

Pancreatoblastoma: a Rare Tumour Accidentally Found Naik V R, Jaafar H, Leow V M, Bhavaraju V M K
Case Report SingaporeSingapore Med Med J 2006; J 2006; 47(3) 47(3) : 232 : 1 Pancreatoblastoma: a rare tumour accidentally found Naik V R, Jaafar H, Leow V M, Bhavaraju V M K ABSTRACT raising the possibility that genetic events on A 15-year-old girl, who was previously well, chromosome 11p might play a role. complained of a mass in the abdomen after The clinical presentations of these tumours a minor motor vehicle accident. Physical are varied. They can present as abdominal pain, and radiological investigations revealed a abdominal mass, diarrhoea, or upper gastrointestinal mass in the body of pancreas containing bleeding. Most of the time, they are asymptomatic. proteinaceous material and multiple The presenting features are highly non-specific and nodules in both lobes of liver. Serological this leads to diagnostic dilemmas. The tumour is investigations for malignancy were normal. slightly more frequent in males, with the median Histopathological examination of the resected age of presentation being five years. Though specimen showed pancreatoblastoma. malignant, these tumours have an indolent course. Pancreatoblastoma is an unusual malignant They can be cured by complete resection alone tumour seen in infants and children although and in cases of unresectable tumours, incomplete rare cases have also been reported in adults. resection and in those with metastatic lesions, They are clinicopathologically distinct from radiotherapy or chemotherapy may be given. The adult pancreatic ductal carcinoma. The prognosis is worse in the presence of synchronous histogenesis, clinical features and treatment or metachronous metastasis and non-resectable options are discussed along with presentation (1) Department of disease at presentation . -

WO 2018/035138 Al 22 February 2018 (22.02.2018) W !P O PCT
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2018/035138 Al 22 February 2018 (22.02.2018) W !P O PCT (51) International Patent Classification: SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, A61L 27/36 (2006.01) A01K 67/027 (2006.01) TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. C12N S/00 (2006.01) C12N 5/077 (2010.01) (84) Designated States (unless otherwise indicated, for every C12N 5/071 (2010.01) G01N 33/50 (2006.01) kind of regional protection available): ARIPO (BW, GH, C12N 5/09 (2010.01) GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, TZ, (21) International Application Number: UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, TJ, PCT/US20 17/046983 TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, DK, EE, ES, FI, FR, GB, GR, HR, HU, IE, IS, IT, LT, LU, LV, (22) International Filing Date: MC, MK, MT, NL, NO, PL, PT, RO, RS, SE, SI, SK, SM, 15 August 2017 (15.08.2017) TR), OAPI (BF, BJ, CF, CG, CI, CM, GA, GN, GQ, GW, (25) Filing Language: English KM, ML, MR, NE, SN, TD, TG). (26) Publication Language: English Published: (30) Priority Data: — with international search report (Art. 21(3)) 62/375,016 15 August 2016 (15.08.2016) US 62/430,015 05 December 2016 (05.12.2016) US (71) Applicants: ORGANOVO, INC. -

Conversion of Morphology of ICD-O-2 to ICD-O-3
NATIONAL INSTITUTES OF HEALTH National Cancer Institute to Neoplasms CONVERSION of NEOPLASMS BY TOPOGRAPHY AND MORPHOLOGY from the INTERNATIONAL CLASSIFICATION OF DISEASES FOR ONCOLOGY, SECOND EDITION to INTERNATIONAL CLASSIFICATION OF DISEASES FOR ONCOLOGY, THIRD EDITION Edited by: Constance Percy, April Fritz and Lynn Ries Cancer Statistics Branch, Division of Cancer Control and Population Sciences Surveillance, Epidemiology and End Results Program National Cancer Institute Effective for cases diagnosed on or after January 1, 2001 TABLE OF CONTENTS Introduction .......................................... 1 Morphology Table ..................................... 7 INTRODUCTION The International Classification of Diseases for Oncology, Third Edition1 (ICD-O-3) was published by the World Health Organization (WHO) in 2000 and is to be used for coding neoplasms diagnosed on or after January 1, 2001 in the United States. This is a complete revision of the Second Edition of the International Classification of Diseases for Oncology2 (ICD-O-2), which was used between 1992 and 2000. The topography section is based on the Neoplasm chapter of the current revision of the International Classification of Diseases (ICD), Tenth Revision, just as the ICD-O-2 topography was. There is no change in this Topography section. The morphology section of ICD-O-3 has been updated to include contemporary terminology. For example, the non-Hodgkin lymphoma section is now based on the World Health Organization Classification of Hematopoietic Neoplasms3. In the process of revising the morphology section, a Field Trial version was published and tested in both the United States and Europe. Epidemiologists, statisticians, and oncologists, as well as cancer registrars, are interested in studying trends in both incidence and mortality. -

TCF-001 TRACK (Target Rare Cancer Knowledge)
TCF-001 TRACK (Target Rare Cancer Knowledge) Cancers not listed here may be enrolled with the approval of the Principal Investigator on a case by case basis. Tier Tumor 1 EPITHELIAL TUMORS OF CERVIX UTERI 2 Squamous cell carcinoma with variants of cervix uteri 3 Squamous carcinoma 3 Squamous cell carcinoma nonkeratinizing, NOS 3 Squamous cell carcinoma keratinizing, NOS 3 Papillary squamous cell carcinoma 3 Papillary carcinoma, NOS 3 Verrucous/Warty carcinoma 3 Basaloid carcinoma 3 Squamous cell carcinoma spindle cell 3 Lymphoepithelial carcinoma 3 Transitional cell carcinoma, NOS 3 Glassy cell carcinoma 2 Adenocarcinoma with variants of cervix uteri 3 Adenocarcinoma, NOS 3 Adenocarcinoma with squamous metaplasia 3 Mucinous adenocarcinoma 3 Clear cell adenocarcinoma, NOS 3 Endometrioid adenocarcinoma, NOS 3 Serous cystadenocarcinoma, NOS 3 Signet ring cell carcinoma 3 Mesonephroma malignant 3 Villous adenocarcinoma 3 Mucinous adenocarcinoma, endocervical type 3 Adenocarcinoma intestinal type 3 Mixed cell adenocarcinoma 2 UnDifferentiateD carcinoma of cervix uteri 1 MIXED EPITHELIAL AND MESENCHYMAL TUMORS OF UTERUS 3 Mullerian mixed tumor 3 Adenosarcoma 1 EPITHELIAL TUMORS OF NASAL CAVITY AND SINUSES 2 Squamous cell carcinoma with variants of nasal cavity and sinuses 3 Squamous carcinoma 3 Verrucous carcinoma 3 Squamous cell carcinoma spindle cell 3 Papillary squamous cell carcinoma 3 Adenosquamous carcinoma Tier Tumor 3 Squamous cell carcinoma, adenoid 3 Basaloid squamous cell carcinoma 2 Lymphoepithelial carcinoma of nasal cavity and -

Non-Commercial Use Only
Monaldi Archives for Chest Disease 2020; volume 90:1398 Cystic fibrohistiocytic tumour of the lung presenting with recurrent pneumothorax: a case report Christos Kakos1, Savvas Lampridis2, Georgios Geropoulos2, Reena Khiroya3, Achilleas Antonopoulos2, Sofoklis Mitsos2, Nikolaos Panagiotopoulos2 1Department of Cardiothoracic Surgery, Royal Victoria Hospital, Belfast; 2Department of Thoracic Surgery, University College London Hospitals NHS Foundation Trust, London; 3Department of Histopathology, University College London Hospitals NHS Foundation Trust, London, UK and was eventually diagnosed with cystic fibrohistiocytic tumour Abstract of the lung. Clinicians should include this disease in the differen- tial diagnosis of pulmonary cystic lesions and be aware of its asso- Cystic fibrohistiocytic tumour of the lung is a very rare patho- ciation with cellular fibrous histiocytoma. Reporting of more logical entity that occurs either as a primary pulmonary neoplasm cases is warranted to further elucidate the natural course of the dis- or as a metastasis from skin lesions called cellular fibrous histio- ease and optimise its management. cytomas. Herein, we present the case of a 19-year old man with a history of recurrent pneumothoraces who was managed surgically only Introduction Correspondence: Christos Kakos, Ulster Hospital, Dundonald, Belfast, Cystic fibrohistiocytic tumour of the lung is an extremely rare BT16 1RH, UK neoplasm. useIt commonly represents metastatic disease from cellu- Tel. +44.7397314648. E-mail: [email protected] lar fibrous histiocytomas, which are benign cutaneous lesions with low-grade malignant potential [1]. Occasionally, it develops as a Keywords: cystic fibrohistiocytic tumour; lung; pneumothorax; cuta- neous fibrohistiocytic tumour; case report. primary lung tumour. Herein, we present the case of a young man with pulmonary cystic fibrohistiocytic tumour who presented with Contributions: CK, conception and design, collection and assembly of recurrent pneumothoraces and received surgical treatment.