Nonductal Neoplasms of the Pancreas
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Problems in Diagnosis Approach for Carcinoma of Pancreatic Head
CASE REPORT Problems in Diagnosis Approach for Carcinoma of Pancreatic Head Ratu Ratih Kusumayanti*, Marcellus Simadibrata**, Murdani Abdullah**, Rino Alvani Gani***, Lies Luthariana* *Department of Internal Medicine, Faculty of Medicine, University of Indonesia Dr. Cipto Mangunkusumo General National Hospital, Jakarta ** Division of Gastroenterology, Department of Internal Medicine, Faculty of Medicine University of Indonesia/Dr. Cipto Mangunkusumo General National Hospital, Jakarta *** Division of Hepatology, Department of Internal Medicine, Faculty of Medicine University of Indonesia/Dr. Cipto Mangunkusumo General National Hospital, Jakarta ABSTRACT Incidences of pancreatic cancer worldwide have been known to be increased. It is the fifth leading cause of death in United State of America. Seventy percent occurs in the head of the pancreas. Major risk factors are related to age, black race, smokers, high-fat diet, chronic pancreatitis, diabetes mellitus and alcohol consumption. Some clinical symptoms such as jaundice, abdominal pain, unexplained weight loss or ascites can occur early or even late in the course of disease. Diagnosing pancreatic cancer sometimes can be difficult, regarding to discrepancy between clinical symptoms and radiological findings. It is important to take good history of the patient, thorough examination, and combine several modalities in diagnosing tumor of pancreatic head. In this case report, a 54 year-old female, came to the hospital with abdominal swelling and jaundice. Physical examination revealed liver and spleen enlargement and edema on both lower extremities. The laboratory result showed increment in Carcinoembryonic Antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) level, without marked increase in bilirubin level. Dilatation of the pancreatic duct was found in this patient, without any sign of bile stone. -

Scientific Framework for Pancreatic Ductal Adenocarcinoma (PDAC)
Scientific Framework for Pancreatic Ductal Adenocarcinoma (PDAC) National Cancer Institute February 2014 1 Table of Contents Executive Summary 3 Introduction 4 Background 4 Summary of the Literature and Recent Advances 5 NCI’s Current Research Framework for PDAC 8 Evaluation and Expansion of the Scientific Framework for PDAC Research 11 Plans for Implementation of Recommended Initiatives 13 Oversight and Benchmarks for Progress 18 Conclusion 18 Links and References 20 Addenda 25 Figure 1: Trends in NCI Funding for Pancreatic Cancer, FY2000-FY2012 Figure 2: NCI PDAC Funding Mechanisms in FY2012 Figure 3: Number of Investigators with at Least One PDAC Relevant R01 Grant FY2000-FY2012 Figure 4: Number of NCI Grants for PDAC Research in FY 2012 Awarded to Established Investigators, New Investigators, and Early Stage Investigators Table 1: NCI Trainees in Pancreatic Cancer Research Appendices Appendix 1: Report from the Pancreatic Cancer: Scanning the Horizon for Focused Invervention Workshop Appendix 2: NCI Investigators and Projects in PDAC Research 2 Scientific Framework for Pancreatic Ductal Carcinoma Executive Summary Significant scientific progress has been made in the last decade in understanding the biology and natural history of pancreatic ductal adenocarcinoma (PDAC); major clinical advances, however, have not occurred. Although PDAC shares some of the characteristics of other solid malignancies, such as mutations affecting common signaling pathways, tumor heterogeneity, development of invasive malignancy from precursor lesions, -

Case Report Metastasis of Pancreatic Cancer Within Primary Colon Cancer by Overtaking the Stromal Microenvironment
Int J Clin Exp Pathol 2018;11(6):3141-3146 www.ijcep.com /ISSN:1936-2625/IJCEP0075771 Case Report Metastasis of pancreatic cancer within primary colon cancer by overtaking the stromal microenvironment Takeo Nakaya1, Hisashi Oshiro1, Takumi Saito2, Yasunaru Sakuma2, Hisanaga Horie2, Naohiro Sata2, Akira Tanaka1 Departments of 1Pathology, 2Surgery, Jichi Medical University, Shimotsuke, Tochigi, Japan Received March 10, 2018; Accepted April 15, 2018; Epub June 1, 2018; Published June 15, 2018 Abstract: We report a unique case of a 74-old man, who presented with double cancers, showing metastasis of pancreatic cancer to colon cancer. Histopathological examination after surgery revealed that the patient had as- cending colon cancer, which metastasized to the liver (pT4N0M1), as well as pancreatic cancer (pT2N1M1) that metastasized to the most invasive portion of the colon cancer, namely the serosal to subserosal layers. Although the mechanisms for this scenario have yet to be elucidated, we speculate that the metastatic pancreatic carcinoma overtook the stromal microenvironment of the colon cancer. Namely, the cancer microenvironment enriched by can- cer-associated fibroblasts, which supported the colon cancer, might be suitable for the invasion and engraftment by pancreatic carcinoma. The similarity of histological appearance might make it difficult to distinguish metastatic pancreatic carcinoma within colon cancer. Furthermore, the metastasis of pancreatic carcinoma in colon carcinoma might be more common, despite it not having been previously reported. Keywords: Cancer metastasis, metastatic pancreatic cancer, colon cancer, double cancer, tumor microenviron- ment Introduction Case report Prevention and control of cancer metastasis is Clinical history one of the most important problems in cancer care [1-4]. -

A Case of Renal Cell Carcinoma Metastasizing to Invasive Ductal Breast Carcinoma Tai-Di Chen, Li-Yu Lee*
Journal of the Formosan Medical Association (2014) 113, 133e136 Available online at www.sciencedirect.com journal homepage: www.jfma-online.com CASE REPORT A case of renal cell carcinoma metastasizing to invasive ductal breast carcinoma Tai-Di Chen, Li-Yu Lee* Department of Pathology, Chang Gung Memorial Hospital and Chang Gung University College of Medicine, Guishan Township, Taoyuan County, Taiwan, ROC Received 12 December 2009; received in revised form 20 May 2010; accepted 1 July 2010 KEYWORDS Tumor-to-tumor metastasis is an uncommon but well-documented phenomenon. We present breast carcinoma; a case of a clear cell renal cell carcinoma (RCC) metastasizing to an invasive ductal carcinoma invasive ductal (IDC)ofthebreast.A74-year-oldwomanwitha past history of clear cell RCC status after carcinoma; radical nephrectomy underwent right modified radical mastectomy for an enlarging breast renal cell carcinoma; mass 3 years after nephrectomy. Histological examination revealed a small focus with distinct tumor-to-tumor morphological features similar to clear cell RCC encased in the otherwise typical IDC. Immu- metastasis nohistochemical studies showed that this focus was positive for CD10 and vimentin, in contrast to the surrounding IDC, which was negative for both markers and positive for Her2/neu. Based on the histological and immunohistochemical features, the patient was diagnosed with metas- tasis of clear cell RCC to the breast IDC. To the best of our knowledge, this is the first reported case of a breast neoplasm as the recipient tumor in tumor-to-tumor metastasis. Copyright ª 2012, Elsevier Taiwan LLC & Formosan Medical Association. All rights reserved. Introduction tumor is renal cell carcinoma (RCC, 38.8%), followed by meningioma (25.4%), and the most frequent donor tumor is The phenomenon of tumor-to-tumor metastasis was first lung cancer (55.8%). -

Rare Pancreatic Tumors
Published online: 2020-04-29 THIEME 64 ReviewRare Pancreatic Article Tumors Choudhari et al. Rare Pancreatic Tumors Amitkumar Choudhari1,2 Pooja Kembhavi1,2 Mukta Ramadwar3,4 Aparna Katdare1,2 Vasundhara Smriti1,2 Akshay D. Baheti1,2 1Department of Radiodiagnosis, Tata Memorial Hospital, Mumbai, Address for correspondence Akshay D. Baheti, MD, Department of Maharashtra, India Radiodiagnosis, Tata Memorial Hospital, Ernest , Borges Marg Parel 2Department of Radiodiagnosis, Homi Bhabha National University, Mumbai 400012, India (e-mail: [email protected]). Mumbai, Maharashtra, India 3Department of Pathology, Tata Memorial Hospital, Mumbai, Maharashtra, India 4Department of Pathology, Homi Bhabha National University, Mumbai, Maharashtra, India J Gastrointestinal Abdominal Radiol ISGAR 2020;3:64–74 Abstract Pancreatic ductal adenocarcinoma, neuroendocrine tumor, and cystic pancreatic neo- plasms are the common pancreatic tumors most radiologists are familiar with. In this Keywords article we review the clinical presentation, pathophysiology, and radiology of rare pan- ► pancreatic cancer creatic neoplasms. While the imaging features are usually nonspecific and diagnosis is ► uncommon based on pathology, the radiology along with patient demographics, history, and lab- ► pancreatoblastoma oratory parameters can often help indicate the diagnosis of an uncommon pancreatic ► acinar cell neoplasm and guide appropriate management in these cases. ► lymphoma Introduction hyperlipasemia may rarely lead to extraabdominal manifes- tations like ectopic subcutaneous fat necrosis and polyarthri- Pancreatic tumors of various histological subtypes can be tis (lipase hypersecretion syndrome).4 encountered in clinical practice, most common being pan- These tumors are hypoenhancing compared with the pan- creatic ductal adenocarcinoma (PDAC), which constitutes creas and are frequently associated with cystic or necrotic 85% of all pancreatic neoplasms.1 Histologically pancreat- areas as well as calcifications5,6 (►Fig. -
Solid Tumour Section Mini Review
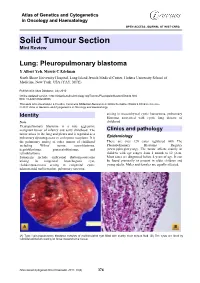
Atlas of Genetics and Cytogenetics in Oncology and Haematology OPEN ACCESS JOURNAL AT INIST-CNRS Solid Tumour Section Mini Review Lung: Pleuropulmonary blastoma Y Albert Yeh, Morris C Edelman North Shore University Hospital, Long Island Jewish Medical Center, Hofstra University School of Medicine, New York, USA (YAY, MCE) Published in Atlas Database: July 2010 Online updated version : http://AtlasGeneticsOncology.org/Tumors/PleuropulmblastomID6040.html DOI: 10.4267/2042/45006 This work is licensed under a Creative Commons Attribution-Noncommercial-No Derivative Works 2.0 France Licence. © 2011 Atlas of Genetics and Cytogenetics in Oncology and Haematology arising in mesenchymal cystic hamartoma, pulmonary Identity blastoma associated with cystic lung disease of Note childhood. Pleuropulmonary blastoma is a rare aggressive malignant tumor of infancy and early childhood. The Clinics and pathology tumor arises in the lung and pleura and is regarded as a pulmonary dysontogenetic or embryonic neoplasm. It is Epidemiology the pulmonary analog of other tumors of childhood There are over 120 cases registered with The including Wilms' tumor, neuroblastoma, Pleuropulmonary Blastoma Registry hepatoblastoma, pancreatoblastoma, and (www.ppbregistry.org). The tumor affects mainly in retinoblastoma. children with age ranges from 1 month to 12 years. Synonyms include embryonal rhabomyosarcoma Most cases are diagnosed before 4 years of age. It can arising in congenital bronchogenic cyst, be found prenatally or present in older children and rhabdomyosarcoma arising in congenital cystic young adults. Males and females are equally affected. adenomatoid malformation, pulmonary sarcoma (A) Type I pleuropulmonary blastoma consists of multiloculated cyst filled with scanty clear serous fluid. (B) The cysts are lined by cuboidal epithelium resting on loose mesenchymal tissue. -

Metastatic Renal Cell Cancer Presenting As a Breast Mass
H & 0 C l i n i C a l C a s e s t u d i e s Metastatic Renal Cell Cancer Presenting as a Breast Mass Neeta Pathe, MD Department of Hematology and Oncology, Allegheny General Hospital, Jane Raymond, MD Pittsburgh, Pennsylvania Alice Ulhoa Cintra, MD introduction a focus of residual DCIS extending to the lateral resec- tion margin. The 2 sentinel lymph nodes examined Metastases to the breast are uncommon, and demand an were benign. Two weeks after her surgery, the patient accurate and prompt diagnosis due to differences in prog- complained of increased swelling on the medial side of nosis and management from primary breast cancer. Here the left breast. This swelling was re-evaluated by a repeat we describe a case of renal cell cancer metastasizing to the ultrasound, which showed an unchanged size of the oval breast 10 years after nephrectomy for the primary tumor. mass and mixed echogenicity. Historically, the prognosis for such a patient has been Preoperatively, a chest X-ray revealed a 6-mm right extremely poor. In the era of novel therapies, however, we lung nodule, and a computed tomography (CT) scan was are now able to provide treatment with an oral agent and recommended for follow-up. The CT scan of the chest, achieve an excellent response. which was performed approximately 3 months after the right lumpectomy, revealed multiple bilateral pulmonary Case study nodules measuring 4–5 mm. Additionally, the lesion in the left breast had increased to 2.7 × 1.9 cm and was suspicious A 64-year-old African American woman with a history for metastatic disease (Figure 1). -

Rare Solid Tumors of the Pancreas As Differential Diagnosis of Pancreatic Adenocarcinoma
JOP. J Pancreas (Online) 2012 May 10; 13(3):268-277. ORIGINAL ARTICLE Rare Solid Tumors of the Pancreas as Differential Diagnosis of Pancreatic Adenocarcinoma Sabine Kersting1, Monika S Janot1, Johanna Munding2, Dominique Suelberg1, Andrea Tannapfel2, Ansgar M Chromik1, Waldemar Uhl1, Uwe Bergmann1 Departments of 1General and Visceral Surgery, St. Josef-Hospital, and 2Pathology; Ruhr-University Bochum. Bochum, Germany ABSTRACT Context Rare solid tumors of the pancreas can be misinterpreted as primary pancreatic cancer. Objective The aim of this study was to report our experience in the treatment of patients with rare tumor lesions of the pancreas and to discuss clinical and pathological characteristics in the context of the role of surgery. Design Data from patients of our prospective data-base with rare benign and malignant tumors of the pancreas, treated in our division from January 2004 to August 2010, were analyzed retrospectively. Results One-thousand and ninety-eight patients with solid tumors of the pancreas underwent pancreatic surgery. In 19 patients (10 women, 9 men) with a mean age of 57 years (range: 20-74 years) rare pancreatic tumors (metastasis, solid pseudopapillary tumor, teratoma, hemangioma, accessory spleen, lymphoepithelial cyst, hamartoma, sarcoidosis, yolk sac tumor) were the reason for surgical intervention. Conclusion If rare benign and malignant pancreatic tumors, intrapancreatic metastasis, as well as pancreatic malformations or other abnormalities, present themselves as solid masses of the pancreas, they constitute an important differential diagnosis to primary pancreatic neoplasia, e.g. pancreatic ductal adenocarcinoma. Clinical imaging techniques cannot always rule out malignancy, thus operative exploration often remains the treatment of choice to provide the correct diagnosis and initiate adequate surgical therapy. -

Large Duct Type Invasive Adenocarcinoma of the Pancreas with Microcystic and Papillary Patterns: a Potential Microscopic Mimic of Non-Invasive Ductal Neoplasia
Modern Pathology (2012) 25, 439–448 & 2012 USCAP, Inc. All rights reserved 0893-3952/12 $32.00 439 Large duct type invasive adenocarcinoma of the pancreas with microcystic and papillary patterns: a potential microscopic mimic of non-invasive ductal neoplasia Pelin Bagci1, Aleodor A Andea2, Olca Basturk3, Kee-Taek Jang4,IpekErbarut5 and Volkan Adsay5 1Department of Pathology, Rize University, School of Medicine, Rize, Turkey; 2Department of Pathology, University of Alabama at Birmingham, Birmingham, AL, USA; 3Department of Pathology, Memorial Sloan-Kettering Cancer Center, New York City, NY, USA; 4Department of Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea and 5Department of Pathology, Emory University, Atlanta, GA, USA A morphological variant of pancreatic ductal adenocarcinoma forming large ductal elements, large duct type ductal adenocarcinoma, is documented and its clinicopathological features are studied. These tumors may have microcystic and papillary growth patterns that closely mimic the non-invasive cystic and papillary pancreatic tumors such as: intraductal papillary-mucinous neoplasia, including the oncocytic variant, mucinous cystic neoplasms, and ducts involved by pancreatic intraepithelial neoplasia. In a review of 230 pancreatectomy specimens with ductal adenocarcinoma, 28 (8%) cases of large duct type ductal adenocarcinomas were identified according to following criteria: more than 50% of the tumor sections available for examination contained infiltrative ducts with a diameter larger than 0.5 mm or had a macroscopically identifiable microcystic pattern. Overall characteristics of large duct type ductal adenocarcinomas were not too different than those of conventional ductal adenocarcinomas, except that there was a slight female predominance in the former (F/M ¼ 2.3). -

Liver, Gallbladder, Bile Ducts, Pancreas
Liver, gallbladder, bile ducts, pancreas Coding issues Otto Visser May 2021 Anatomy Liver, gallbladder and the proximal bile ducts Incidence of liver cancer in Europe in 2018 males females Relative survival of liver cancer (2000 10% 15% 20% 25% 30% 35% 40% 45% 50% 0% 5% Bulgaria Latvia Estonia Czechia Slovakia Malta Denmark Croatia Lithuania N Ireland Slovenia Wales Poland England Norway Scotland Sweden Netherlands Finland Iceland Ireland Austria Portugal EUROPE - Germany 2007) Spain Switzerland France Belgium Italy five year one year Liver: topography • C22.1 = intrahepatic bile ducts • C22.0 = liver, NOS Liver: morphology • Hepatocellular carcinoma=HCC (8170; C22.0) • Intrahepatic cholangiocarcinoma=ICC (8160; C22.1) • Mixed HCC/ICC (8180; TNM: C22.1; ICD-O: C22.0) • Hepatoblastoma (8970; C22.0) • Malignant rhabdoid tumour (8963; (C22.0) • Sarcoma (C22.0) • Angiosarcoma (9120) • Epithelioid haemangioendothelioma (9133) • Embryonal sarcoma (8991)/rhabdomyosarcoma (8900-8920) Morphology*: distribution by sex (NL 2011-17) other other ICC 2% 3% 28% ICC 56% HCC 41% HCC 70% males females * Only pathologically confirmed cases Liver cancer: primary or metastatic? Be aware that other and unspecified morphologies are likely to be metastatic, unless there is evidence of the contrary. For example, primary neuro-endocrine tumours (including small cell carcinoma) of the liver are extremely rare. So, when you have a diagnosis of a carcinoid or small cell carcinoma in the liver, this is probably a metastatic tumour. Anatomy of the bile ducts Gallbladder -

Input Data Dictionary Statistics Canada Health Statistics Division
Component of Statistics Canada Catalogue no. 82-225-XIE2005000 ISSN: 1715-2100 O Canadian Cancer Registry Manual 2005 Input Data Dictionary Statistics Canada Health Statistics Division Canadian Cancer Registry Input Data Dictionary Published by authority of the Minister responsible for Statistics Canada © Minister of Industry, 2003 Material appearing in this publication may be reproduced or copied without permission; however, the following citation to indicate the source must be used: "Data sources: Statistics Canada, Canadian Cancer Registry, Ottawa, 2003." February 2005 Catalogue no. 84-601-XIE Frequency: Irregular Ottawa La version française de cette publication est disponible gratuitement sur le site Internet de Statistique Canada (no 84-601-XIF au catalogue). Note of Appreciation Canada owes the success of its statistical system to a long-standing partnership between Statistics Canada, the citizens of Canada, its businesses, governments and other institutions. Accurate and timely statistical information could not be produced without their continued cooperation and goodwill. Canadian Cancer Registry – Input Data Dictionary Table of Contents Page 1.0 Introduction........................................................................................................................1 1.1 Content of Input Data Dictionary ............................................................................1 1.2 CCR Overview.........................................................................................................2 1.2.1 Canadian Cancer -

Enlarging Nodule on the Nipple
PHOTO CHALLENGE Enlarging Nodule on the Nipple Caren Waintraub, MD; Brianne Daniels, DO; Shari R. Lipner, MD, PhD Eligible for 1 MOC SA Credit From the ABD This Photo Challenge in our print edition is eligible for 1 self-assessment credit for Maintenance of Certification from the American Board of Dermatology (ABD). After completing this activity, diplomates can visit the ABD website (http://www.abderm.org) to self-report the credits under the activity title “Cutis Photo Challenge.” You may report the credit after each activity is completed or after accumulating multiple credits. A healthy 48-year-old woman presented with a growth on the right nipple that had been slowly enlarging over the last few months. She initially noticed mild swellingcopy in the area that persisted and formed a soft lump. She described mild pain with intermittent drainage but no bleeding. Her medical history was unremarkable, including a negativenot personal and family history of breast and skin cancer. She was taking no medications prior to development of the mass. She had no recent history of pregnancy or breastfeeding. A mammo- Dogram and breast ultrasound were not concerning for carcinoma. Physical examination showed a soft, exophytic, mildly tender, pink nodule on the right nipple that measured 12×7 mm; no drainage, bleeding, or ulceration was present. The surround- ing skin of the areola and breast demonstrated no clinical changes. The contralateral breast, areola, and nipple were unaffected. The patient had no appreciable axillary or cervical lymphadenopathy. A deep shave biopsy of the noduleCUTIS was performed and sent for histopathologic examination.